G-quadruplexes Stabilization Upregulates CCN1 and Accelerates Aging in Cultured Cerebral Endothelial Cells
- PMID: 35822045
- PMCID: PMC9261356
- DOI: 10.3389/fragi.2021.797562
G-quadruplexes Stabilization Upregulates CCN1 and Accelerates Aging in Cultured Cerebral Endothelial Cells
Abstract
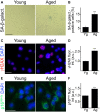
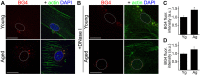
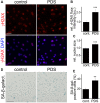
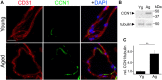
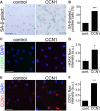
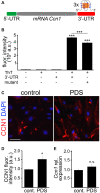
Senescence in the cerebral endothelium has been proposed as a mechanism that can drive dysfunction of the cerebral vasculature, which precedes vascular dementia. Cysteine-rich angiogenic inducer 61 (Cyr61/CCN1) is a matricellular protein secreted by cerebral endothelial cells (CEC). CCN1 induces senescence in fibroblasts. However, whether CCN1 contributes to senescence in CEC and how this is regulated requires further study. Aging has been associated with the formation of four-stranded Guanine-quadruplexes (G4s) in G-rich motifs of DNA and RNA. Stabilization of the G4 structures regulates transcription and translation either by upregulation or downregulation depending on the gene target. Previously, we showed that aged mice treated with a G4-stabilizing compound had enhanced senescence-associated (SA) phenotypes in their brains, and these mice exhibited enhanced cognitive deficits. A sequence in the 3'-UTR of the human CCN1 mRNA has the ability to fold into G4s in vitro. We hypothesize that G4 stabilization regulates CCN1 in cultured primary CEC and induces endothelial senescence. We used cerebral microvessel fractions and cultured primary CEC from young (4-months old, m/o) and aged (18-m/o) mice to determine CCN1 levels. SA phenotypes were determined by high-resolution fluorescence microscopy in cultured primary CEC, and we used Thioflavin T to recognize RNA-G4s for fluorescence spectra. We found that cultured CEC from aged mice exhibited enhanced levels of SA phenotypes, and higher levels of CCN1 and G4 stabilization. In cultured CEC, CCN1 induced SA phenotypes, such as SA β-galactosidase activity, and double-strand DNA damage. Furthermore, CCN1 levels were upregulated by a G4 ligand, and a G-rich motif in the 3'-UTR of the Ccn1 mRNA was folded into a G4. In conclusion, we demonstrate that CCN1 can induce senescence in cultured primary CEC, and we provide evidence that G4 stabilization is a novel mechanism regulating the SASP component CCN1.
Keywords: CCN1; G-quadruplex; aging; endothelial cells; senescence.
Copyright © 2022 Noh, Blasco-Conesa, Lai, Ganesh, Urayama, Moreno-Gonzalez, Marrelli, McCullough and Moruno-Manchon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
G-quadruplexes and associated proteins in aging and Alzheimer's disease.Front Aging. 2023 Jun 1;4:1164057. doi: 10.3389/fragi.2023.1164057. eCollection 2023. Front Aging. 2023. PMID: 37323535 Free PMC article. Review.
-
Iron overload induces cerebral endothelial senescence in aged mice and in primary culture in a sex-dependent manner.Aging Cell. 2023 Nov;22(11):e13977. doi: 10.1111/acel.13977. Epub 2023 Sep 7. Aging Cell. 2023. PMID: 37675802 Free PMC article.
-
Pirh2-dependent DNA damage in neurons induced by the G-quadruplex ligand pyridostatin.J Biol Chem. 2023 Oct;299(10):105157. doi: 10.1016/j.jbc.2023.105157. Epub 2023 Aug 12. J Biol Chem. 2023. PMID: 37579947 Free PMC article.
-
Aging increases CCN1 expression leading to muscle senescence.Am J Physiol Cell Physiol. 2014 Jan 1;306(1):C28-36. doi: 10.1152/ajpcell.00066.2013. Epub 2013 Nov 6. Am J Physiol Cell Physiol. 2014. PMID: 24196529 Free PMC article.
-
Structurally diverse G-quadruplexes as the noncanonical nucleic acid drug target for live cell imaging and antibacterial study.Chem Commun (Camb). 2023 Feb 2;59(11):1415-1433. doi: 10.1039/d2cc05945b. Chem Commun (Camb). 2023. PMID: 36636928 Review.
Cited by
-
Involvement of Matricellular Proteins in Cellular Senescence: Potential Therapeutic Targets for Age-Related Diseases.Int J Mol Sci. 2024 Jun 15;25(12):6591. doi: 10.3390/ijms25126591. Int J Mol Sci. 2024. PMID: 38928297 Free PMC article. Review.
-
Benefits of equilibrium between microbiota- and host-derived ligands of the aryl hydrocarbon receptor after stroke in aged male mice.Nat Commun. 2025 Feb 19;16(1):1767. doi: 10.1038/s41467-025-57014-2. Nat Commun. 2025. PMID: 39971928 Free PMC article.
-
An Updated Overview on the Role of Small Molecules and Natural Compounds in the "Young Science" of Rejuvenation.Antioxidants (Basel). 2023 Jan 27;12(2):288. doi: 10.3390/antiox12020288. Antioxidants (Basel). 2023. PMID: 36829846 Free PMC article. Review.
-
Cellular senescence and the blood-brain barrier: Implications for aging and age-related diseases.Exp Biol Med (Maywood). 2023 May;248(5):399-411. doi: 10.1177/15353702231157917. Epub 2023 Apr 3. Exp Biol Med (Maywood). 2023. PMID: 37012666 Free PMC article. Review.
-
G-quadruplexes and associated proteins in aging and Alzheimer's disease.Front Aging. 2023 Jun 1;4:1164057. doi: 10.3389/fragi.2023.1164057. eCollection 2023. Front Aging. 2023. PMID: 37323535 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

