Transcriptional and Post-Transcriptional Regulations of Amyloid-β Precursor Protein ( APP ) mRNA
- PMID: 35822056
- PMCID: PMC9261399
- DOI: 10.3389/fragi.2021.721579
Transcriptional and Post-Transcriptional Regulations of Amyloid-β Precursor Protein ( APP ) mRNA
Abstract
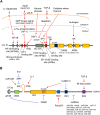
Alzheimer's disease (AD) is an age-associated neurodegenerative disorder characterized by progressive impairment of memory, thinking, behavior, and dementia. Based on ample evidence showing neurotoxicity of amyloid-β (Aβ) aggregates in AD, proteolytically derived from amyloid precursor protein (APP), it has been assumed that misfolding of Aβ plays a crucial role in the AD pathogenesis. Additionally, extra copies of the APP gene caused by chromosomal duplication in patients with Down syndrome can promote AD pathogenesis, indicating the pathological involvement of the APP gene dose in AD. Furthermore, increased APP expression due to locus duplication and promoter mutation of APP has been found in familial AD. Given this background, we aimed to summarize the mechanism underlying the upregulation of APP expression levels from a cutting-edge perspective. We first reviewed the literature relevant to this issue, specifically focusing on the transcriptional regulation of APP by transcription factors that bind to the promoter/enhancer regions. APP expression is also regulated by growth factors, cytokines, and hormone, such as androgen. We further evaluated the possible involvement of post-transcriptional regulators of APP in AD pathogenesis, such as RNA splicing factors. Indeed, alternative splicing isoforms of APP are proposed to be involved in the increased production of Aβ. Moreover, non-coding RNAs, including microRNAs, post-transcriptionally regulate the APP expression. Collectively, elucidation of the novel mechanisms underlying the upregulation of APP would lead to the development of clinical diagnosis and treatment of AD.
Keywords: Alzheimer’s disease; RNA-binding protein; alternative splicing; amyloid precursor protein; dementia; microRNA; post-transcription; transcription.
Copyright © 2021 Sato, Takayama, Hashimoto and Inoue.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Post-transcriptional regulation of amyloid precursor protein by microRNAs and RNA binding proteins.Commun Integr Biol. 2010 Nov;3(6):499-503. doi: 10.4161/cib.3.6.13172. Epub 2010 Nov 1. Commun Integr Biol. 2010. PMID: 21331224 Free PMC article.
-
NGF and the Amyloid Precursor Protein in Alzheimer's Disease: From Molecular Players to Neuronal Circuits.Adv Exp Med Biol. 2021;1331:145-165. doi: 10.1007/978-3-030-74046-7_10. Adv Exp Med Biol. 2021. PMID: 34453297 Review.
-
ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer's Neuronal Pathology.J Neurosci. 2016 Mar 30;36(13):3848-59. doi: 10.1523/JNEUROSCI.3757-15.2016. J Neurosci. 2016. PMID: 27030769 Free PMC article.
-
Amyloid precursor protein, an androgen-regulated gene, is targeted by RNA-binding protein PSF/SFPQ in neuronal cells.Genes Cells. 2019 Nov;24(11):719-730. doi: 10.1111/gtc.12721. Epub 2019 Oct 15. Genes Cells. 2019. PMID: 31541592
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Role of RNA binding proteins of the Drosophila behavior and human splicing (DBHS) family in health and cancer.RNA Biol. 2024 Jan;21(1):1-17. doi: 10.1080/15476286.2024.2332855. Epub 2024 Mar 29. RNA Biol. 2024. PMID: 38551131 Free PMC article. Review.
-
ERRα and ERRγ coordinate expression of genes associated with Alzheimer's disease, inhibiting DKK1 to suppress tau phosphorylation.Proc Natl Acad Sci U S A. 2024 Sep 10;121(37):e2406854121. doi: 10.1073/pnas.2406854121. Epub 2024 Sep 4. Proc Natl Acad Sci U S A. 2024. PMID: 39231208 Free PMC article.
-
Expression and function of estrogen receptors and estrogen-related receptors in the brain and their association with Alzheimer's disease.Front Endocrinol (Lausanne). 2023 Jul 4;14:1220150. doi: 10.3389/fendo.2023.1220150. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37469978 Free PMC article. Review.
-
Association of GLOD4 with Alzheimer's Disease in Humans and Mice.J Alzheimers Dis. 2024;101(3):823-834. doi: 10.3233/JAD-240512. J Alzheimers Dis. 2024. PMID: 39302370 Free PMC article.
-
Stress granules sequester Alzheimer's disease-associated gene transcripts and regulate disease-related neuronal proteostasis.Aging (Albany NY). 2023 May 22;15(10):3984-4011. doi: 10.18632/aging.204737. Epub 2023 May 22. Aging (Albany NY). 2023. PMID: 37219408 Free PMC article.
References
-
- Akao Y., Marukawa O., Morikawa H., Nakao K., Kamei M., Hachiya T., et al. (1995). The Rck/p54 Candidate Proto-Oncogene Product Is a 54-kilodalton D-E-A-D Box Protein Differentially Expressed in Human and Mouse Tissues. Cancer Res. 55, 3444–3449. - PubMed
-
- Bandyopadhyay S., Cahill C., Balleidier A., Huang C., Lahiri D. K., Huang X., et al. (2013). Novel 5′ Untranslated Region Directed Blockers of Iron-Regulatory Protein-1 Dependent Amyloid Precursor Protein Translation: Implications for Down Syndrome and Alzheimer's Disease. PLoS One 8, e65978. 10.1371/journal.pone.0065978 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

