Preference Testing in Medical Devices: Current Framework and Regulatory Gaps
- PMID: 35822064
- PMCID: PMC9271283
- DOI: 10.2147/MDER.S368420
Preference Testing in Medical Devices: Current Framework and Regulatory Gaps
Abstract
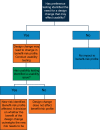
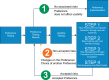
Preference testing is a valuable source of information that can be provided by both healthcare professionals (HCPs) and patients (users). It can be used to improve the design and development of medical devices by feeding into device usability and, ultimately, risk management. Furthermore, it can aid with selecting the most appropriate clinical endpoints to be used in the clinical evaluation of a device and increase patient engagement by incorporating patient-relevant outcomes. Preference testing is widely conducted in the food industry but is not widespread in the medical field due to limited guidelines and a lack of regulatory framework. As such, manufacturers may be unaware of the benefits of preference testing and fail to take full advantage of it, or conversely, may use inappropriate methodology and/or analyses and consequently fail to collect meaningful data. In this position paper, we aim to highlight the benefits and uses of preference testing, along with potential methods that could be used for preference testing of medical devices. A key step towards the wider implementation of preference testing in medical devices is for the publication of international standards and guidelines for the collection, assessment, and implementation of preference data into the life cycle of a medical device.
Keywords: PPI; medical device; patient preference; preference testing.
© 2022 Lewis et al.
Conflict of interest statement
Dr Amie Smirthwaite reports on working for a medical devices consultancy. The authors report no other conflicts of interest in this work.
Figures
References
-
- E2943-15, A. Standard guide for two-sample acceptance and preference testing with consumers; 2021; Available from: https://www.astm.org/e2943-15r21.html. Accessed June 16, 2022.
-
- Hein K, Jaeger SR, Tom Carr B, et al. Comparison of five common acceptance and preference methods. Food Qual Prefer. 2008;19(7):651–661. doi: 10.1016/j.foodqual.2008.06.001 - DOI
-
- FDA, Virtual ISPOR-FDA Summit 2020. Using patient preference information in medical device regulatory decisions: benefit-risk and beyond; 2020.
-
- Ellis BH. Acceptance and consumer preference testing. J Dairy Sci. 1969;52(6):823–831. doi: 10.3168/jds.S0022-0302(69)86658-0 - DOI
-
- Zbrozek A, Hebert J, Gogates G, et al. validation of electronic systems to collect patient-reported outcome (PRO) data—recommendations for clinical trial teams: report of the ISPOR ePRO systems validation good research practices task force. Value Health. 2013;16(4):480–489. doi: 10.1016/j.jval.2013.04.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials