Combined isobutyryl-CoA and multiple acyl-CoA dehydrogenase deficiency in a boy with altered riboflavin homeostasis
- PMID: 35822092
- PMCID: PMC9259400
- DOI: 10.1002/jmd2.12292
Combined isobutyryl-CoA and multiple acyl-CoA dehydrogenase deficiency in a boy with altered riboflavin homeostasis
Abstract
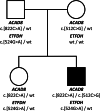
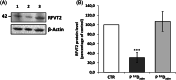
In this report, we describe the case of an 11-year-old boy, who came to our attention for myalgia and muscle weakness, associated with inappetence and vomiting. Hypertransaminasemia was also noted, with ultrasound evidence of hepatomegaly. Biochemical investigations revealed acylcarnitine and organic acid profiles resembling those seen in MADD, that is, multiple acyl-CoA dehydrogenase deficiencies (OMIM #231680) a rare inherited disorder of fatty acids, amino acids, and choline metabolism. The patient carried a single pathogenetic variant in the ETFDH gene (c.524G>A, p.Arg175His) and no pathogenetic variant in the riboflavin (Rf) homeostasis related genes (SLC52A1, SLC52A2, SLC52A3, SLC25A32, FLAD1). Instead, compound heterozygosity was found in the ACAD8 gene (c.512C>G, p.Ser171Cys; c.822C>A, p.Asn274Lys), coding for isobutyryl-CoA dehydrogenase (IBD), whose pathogenic variants are associated to IBD deficiency (OMIM #611283), a rare autosomal recessive disorder of valine catabolism. The c.822C>A was never previously described in a patient. Subsequent further analyses of Rf homeostasis showed reduced levels of flavins in plasma and altered FAD-dependent enzymatic activities in erythrocytes, as well as a significant reduction in the level of the plasma membrane Rf transporter 2 in erythrocytes. The observed Rf/flavin scarcity in this patient, possibly associated with a decreased ETF:QO efficiency might be responsible for the observed MADD-like phenotype. The patient's clinical picture improved after supplementation of Rf, l-carnitine, Coenzyme Q10, and also 3OH-butyrate. This report demonstrates that, even in the absence of genetic defects in genes involved in Rf homeostasis, further targeted molecular analysis may reveal secondary and possibly treatable biochemical alterations in this pattern.
Keywords: ACAD8; ETFDH; IBDD; MADD; RFVT2; riboflavin.
© 2022 The Authors. JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ikeda Y, Okamura‐Ikeda K, Tanaka K. Purification and characterization of short‐chain, medium‐chain, and long‐chain acyl‐CoA dehydrogenases from rat liver mitochondria. Isolation of the holo‐ and apoenzymes and conversion of the apoenzyme to the holoenzyme. J Biol Chem. 1985;260(2):1311‐1325. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

