Comparative analyses of eighteen rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation
- PMID: 35822105
- PMCID: PMC9271059
- DOI: 10.1038/s43856-022-00147-y
Comparative analyses of eighteen rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation
Abstract
Background: Rapid antigen (RA) tests are being increasingly employed to detect SARS-CoV-2 infections in quarantine and surveillance. Prior research has focused on RT-PCR testing, a single RA test, or generic diagnostic characteristics of RA tests in assessing testing strategies.
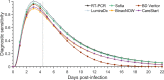
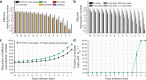
Methods: We have conducted a comparative analysis of the post-quarantine transmission, the effective reproduction number during serial testing, and the false-positive rates for 18 RA tests with emergency use authorization from The United States Food and Drug Administration and an RT-PCR test. To quantify the extent of transmission, we developed an analytical mathematical framework informed by COVID-19 infectiousness, test specificity, and temporal diagnostic sensitivity data.
Results: We demonstrate that the relative effectiveness of RA tests and RT-PCR testing in reducing post-quarantine transmission depends on the quarantine duration and the turnaround time of testing results. For quarantines of two days or shorter, conducting a RA test on exit from quarantine reduces onward transmission more than a single RT-PCR test (with a 24-h delay) conducted upon exit. Applied to a complementary approach of performing serial testing at a specified frequency paired with isolation of positives, we have shown that RA tests outperform RT-PCR with a 24-h delay. The results from our modeling framework are consistent with quarantine and serial testing data collected from a remote industry setting.
Conclusions: These RA test-specific results are an important component of the tool set for policy decision-making, and demonstrate that judicious selection of an appropriate RA test can supply a viable alternative to RT-PCR in efforts to control the spread of disease.
Keywords: Epidemiology; Infectious diseases; Population screening.
Plain language summary
Previous research on SARS-CoV-2 infection has determined optimal timing for testing in quarantine and the utility of different frequencies of testing for infection surveillance using RT-PCR and generalized rapid antigen tests. However, these strategies can depend on the specific rapid antigen test used. By examining 18 rapid antigen tests, we demonstrate that a single rapid antigen test performs better than RT-PCR when quarantines are two days or less in duration. In the context of infection surveillance, the ability of a rapid antigen test to provide results quickly counteracts its lower sensitivity with potentially more false positives. Our findings indicate that rapid antigen tests can be a suitable alternative to RT-PCR for application in quarantine and infection surveillance.
© The Author(s) 2022.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


Similar articles
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
-
Optimal test-assisted quarantine strategies for COVID-19.medRxiv [Preprint]. 2021 Feb 22:2020.11.06.20222398. doi: 10.1101/2020.11.06.20222398. medRxiv. 2021. Update in: BMJ Open. 2021 Jul 16;11(7):e050473. doi: 10.1136/bmjopen-2021-050473. PMID: 33655268 Free PMC article. Updated. Preprint.
-
Reducing COVID-19 quarantine with SARS-CoV-2 testing: a simulation study.BMJ Open. 2021 Jul 16;11(7):e050473. doi: 10.1136/bmjopen-2021-050473. BMJ Open. 2021. PMID: 34272225 Free PMC article.
-
Optimal COVID-19 quarantine and testing strategies.medRxiv [Preprint]. 2020 Nov 30:2020.10.27.20211631. doi: 10.1101/2020.10.27.20211631. medRxiv. 2020. Update in: Nat Commun. 2021 Jan 7;12(1):356. doi: 10.1038/s41467-020-20742-8. PMID: 33173923 Free PMC article. Updated. Preprint.
-
Travel-related control measures to contain the COVID-19 pandemic: a rapid review.Cochrane Database Syst Rev. 2020 Oct 5;10:CD013717. doi: 10.1002/14651858.CD013717. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 25;3:CD013717. doi: 10.1002/14651858.CD013717.pub2. PMID: 33502002 Updated.
Cited by
-
Sex, Age, and Comorbidities Are Associated with SARS-CoV-2 Infection, COVID-19 Severity, and Fatal Outcome in a Mexican Population: A Retrospective Multi-Hospital Study.J Clin Med. 2023 Apr 3;12(7):2676. doi: 10.3390/jcm12072676. J Clin Med. 2023. PMID: 37048758 Free PMC article.
-
Terminating pandemics with smartwatches.PNAS Nexus. 2025 Mar 4;4(3):pgaf044. doi: 10.1093/pnasnexus/pgaf044. eCollection 2025 Mar. PNAS Nexus. 2025. PMID: 40045996 Free PMC article.
-
Clinical accuracy of instrument-based SARS-CoV-2 antigen diagnostic tests: a systematic review and meta-analysis.Virol J. 2024 Apr 29;21(1):99. doi: 10.1186/s12985-024-02371-5. Virol J. 2024. PMID: 38685117 Free PMC article.
-
A Narrative Overview of Coronavirus Infection: Clinical Signs and Symptoms, Viral Entry and Replication, Treatment Modalities, and Management.Curr Top Med Chem. 2024;24(21):1883-1916. doi: 10.2174/0115680266296095240529114058. Curr Top Med Chem. 2024. PMID: 38859776 Review.
-
Modelling the impact of timelines of testing and isolation on disease control.Infect Dis Model. 2023 Mar;8(1):58-71. doi: 10.1016/j.idm.2022.11.008. Epub 2022 Nov 28. Infect Dis Model. 2023. PMID: 36467718 Free PMC article.
References
-
- Haseltine, W. A. Even With A Vaccine, We Still Need Rapid Tests To End Covid-19. Forbeshttps://www.forbes.com/sites/williamhaseltine/2020/12/11/even-with-a-vac... (2020).
-
- Irfan, U. Why the decline in US Covid-19 testing is so alarming. Voxhttps://www.vox.com/22321794/covid-19-testing-test-vaccine-coronavirus-v... (2021).
-
- Thomas, N. & Deidre McPhillips, C. N. N. ‘You’re contradicting yourself’: Tapper presses governor on vaccinations. https://www.cnn.com/videos/politics/2021/06/06/tate-reeves-mississippi-v... (2021).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

