Cholestasis alters brain lipid and bile acid composition and compromises motor function in neonatal piglets
- PMID: 35822260
- PMCID: PMC9277266
- DOI: 10.14814/phy2.15368
Cholestasis alters brain lipid and bile acid composition and compromises motor function in neonatal piglets
Abstract
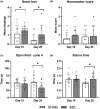
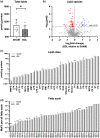
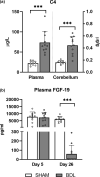
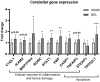
Infants with neonatal cholestasis are prone to neurodevelopmental deficits, however, the underlying pathogenesis is unclear. Lipid malabsorption and accumulation of potentially neurotoxic molecules in the blood such as bile acids are important yet relatively unexplored pathways. Here, we developed a translational piglet model to understand how the molecular bile acid and lipid composition of the brain is affected by this disease and relates to motor function. Piglets (8-days old) had bile duct ligation or sham surgery and were fed a formula diet for 3 weeks. Alongside sensory-motor deficits observed in bile duct-ligated animals, we found a shift toward a more hydrophilic and conjugated bile acid profile in the brain. Additionally, comprehensive lipidomics of the cerebellum revealed a decrease in total lipids including phosphatidylinositols and phosphatidylserines and increases in lysophospholipid species. This was paralleled by elevated cerebellar expression of genes related to inflammation and tissue damage albeit without significant impact on the brain transcriptome. This study offers new insights into the developing brain's molecular response to neonatal cholestasis indicating that bile acids and lipids may contribute in mediating motor deficits.
Keywords: bile acids; brain; cholestasis; lipids; motor skills.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Bile acids content in brain of common duct ligated rats.Ann Hepatol. 2012 Nov-Dec;11(6):930-4. Ann Hepatol. 2012. PMID: 23111582
-
Intravenous fish oil emulsion attenuates total parenteral nutrition-induced cholestasis in newborn piglets.Pediatr Res. 1999 Feb;45(2):202-8. doi: 10.1203/00006450-199902000-00008. Pediatr Res. 1999. PMID: 10022591
-
[Changes in bile acid composition and hepatic microsomal membrane lipid fluidity in bile duct-ligated rat--ESR spin label study].Nihon Shokakibyo Gakkai Zasshi. 1988 Mar;85(3):756. Nihon Shokakibyo Gakkai Zasshi. 1988. PMID: 2838660 Japanese. No abstract available.
-
Bile salts and cholestasis.Dig Liver Dis. 2010 Jun;42(6):409-18. doi: 10.1016/j.dld.2010.03.015. Dig Liver Dis. 2010. PMID: 20434968 Review.
-
Bile Acids in Cholestasis and its Treatment.Ann Hepatol. 2017 Nov;16(Suppl. 1: s3-105.):s53-s57. doi: 10.5604/01.3001.0010.5497. Ann Hepatol. 2017. PMID: 29080340 Review.
Cited by
-
Towards a model of biliary atresia - Pilot feasibility study in newborn piglets.Biochem Biophys Rep. 2023 May 23;34:101487. doi: 10.1016/j.bbrep.2023.101487. eCollection 2023 Jul. Biochem Biophys Rep. 2023. PMID: 37265596 Free PMC article.
-
The bile acid metabolome in umbilical cord blood and meconium of healthy newborns: distinct characteristics and implications.PeerJ. 2024 Dec 13;12:e18506. doi: 10.7717/peerj.18506. eCollection 2024. PeerJ. 2024. PMID: 39686994 Free PMC article.
-
Insulin-like growth factor 1 supplementation supports motor coordination and affects myelination in preterm pigs.Front Neurosci. 2023 Jun 19;17:1205819. doi: 10.3389/fnins.2023.1205819. eCollection 2023. Front Neurosci. 2023. PMID: 37404461 Free PMC article.
-
Automated procedure to detect subtle motor alterations in the balance beam test in a mouse model of early Parkinson's disease.Sci Rep. 2024 Jan 9;14(1):862. doi: 10.1038/s41598-024-51225-1. Sci Rep. 2024. PMID: 38195974 Free PMC article.
References
-
- Almeida, R. , Pauling, J. K. , Sokol, E. , Hannibal‐Bach, H. K. , & Ejsing, C. S. (2015). Comprehensive lipidome analysis by shotgun Lipidomics on a hybrid quadrupole‐orbitrap‐linear ion trap mass spectrometer. Journal of the American Society for Mass Spectrometry, 26, 133–148. 10.1007/s13361-014-1013-x - DOI - PubMed
-
- Balduini, W. , Murphy, S. D. , & Costa, L. G. (1987). Developmental changes in muscarinic receptor‐stimulated phosphoinositide metabolism in rat brain. The Journal of Pharmacology and Experimental Therapeutics, 241, 421–427. - PubMed
-
- Bérubé, N. G. , Swanson, X. H. , Bertram, M. J. , Kittle, J. D. , Didenko, V. , Baskin, D. S. , Smith, J. R. , & Pereira‐Smith, O. M. (1999). Cloning and characterization of CRF, a novel C1q‐related factor, expressed in areas of the brain involved in motor function. Molecular Brain Research, 63, 233–240. 10.1016/S0169-328X(98)00278-2 - DOI - PubMed
-
- Bolliger, M. F. , Martinelli, D. C. , & Südhof, T. C. (2011). The cell‐adhesion G protein‐coupled receptor BAI3 is a high‐affinity receptor for C1q‐like proteins. Proceedings of the National Academy of Sciences of the United States of America, 108, 2534–2539. 10.1073/pnas.1019577108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

