Pragmatic randomized trial assessing the impact of digital health technology on quality of life in patients with heart failure: Design, rationale and implementation
- PMID: 35822275
- PMCID: PMC9346973
- DOI: 10.1002/clc.23848
Pragmatic randomized trial assessing the impact of digital health technology on quality of life in patients with heart failure: Design, rationale and implementation
Abstract
Background: Self-care and patient engagement are important elements of heart failure (HF) care, endorsed in the guidelines. Digital health tools may improve quality of life (QOL) in HF patients by promoting care, knowledge, and engagement. This manuscript describes the rationale and challenges of the design and implementation of a pragmatic randomized controlled trial to evaluate the efficacy of three digital health technologies in improving QOL for patients with HF.
Hypothesis: We hypothesize that digital health interventions will improve QOL of HF patients through the early detection of warning signs of disease exacerbation, the opportunity of self-tracking symptoms, and the education provided, which enhances patient empowerment.
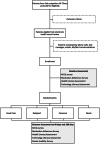
Methods: Using a fully electronic enrollment and consent platform, the trial will randomize 200 patients across HF clinics in the Yale New Haven Health system to receive either usual care or one of three digital technologies designed to promote self-management and provide critical data to clinicians. The primary outcome is the change in QOL as assessed by the Kansas City Cardiomyopathy Questionnaire at 3 months.
Results: First enrollment occurred in September 2021. Recruitment was anticipated to last 6-8 months and participants were followed for 6 months after randomization. Our recruitment efforts have highlighted the large digital divide in our population of interest.
Conclusion: Assessing clinical outcomes, patient usability, and ease of clinical integration of digital technologies will be beneficial in determining the feasibility of the integration of such technologies into the healthcare system.
Keywords: digital health technology; heart failure.
© 2022 The Authors. Clinical Cardiology published by Wiley Periodicals LLC.
Conflict of interest statement
Francis P. Wilson: Founder of Efference, LLC, a medical communications company. Receives support from NIH R01DK113191, R01HS027626, P30DK079310. Nihar Desai: Under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures used for public reporting and pay for performance programs. Reports research grants and consulting for Amgen, Astra Zeneca, Boehringer Ingelheim, Cytokinetics, MyoKardia, Relypsa, Novartis, and SCPharmaceuticals.
Figures

References
-
- Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1‐20. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2017;70(6):776‐803. - PubMed
-
- Writing C, Maddox TM, Januzzi JL Jr., et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772‐810. - PubMed
-
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993‐1004. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

