Assessing the validity and interpretability of the Simplified Psoriasis Index in Tunisian patients
- PMID: 35822332
- PMCID: PMC9002850
Assessing the validity and interpretability of the Simplified Psoriasis Index in Tunisian patients
Abstract
Introduction: Multiple scores have been developed to assess the severity of psoriasis, but these scores have many limitations. The Simplified Psoriasis Index (SPI) is a summary score with separate components for current severity (SPI-s), psychosocial impact (SPI-p), and past history and interventions (SPI-i). It is available in two similar versions: proSPI and saSPI.
Aim: To assess the validity of the SPI by studying its correlation to the benchmark scores in Tunisian patients.
Methods: It was a prospective bicentric study including 80 patients with plaque psoriasis.
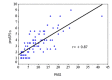
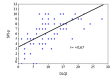
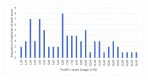
Results: The median PASI was 7.6 and the median DLQI was 9. The median proSPI-s was 6 and the median saSPI-s was 8. The median SPI-p was 7. The median SPI-i was 2. There was a strong correlation between the proSPI-s and PASI (r=0.87) and between the proSPI-s and saSPI-s (r=0.82). There was a medium correlation between saSPI-s and PASI (r=0.70) and between SPI-p and DLQI (r=0.67). The threshold value for proSPI-s and saSPI-s was 7.25. The threshold value for SPI-p was 6.5.
Conclusion: The SPI aims to provide a concise but global measure of the severity and impact of psoriasis on quality of life. The use of SPI has several advantages: the simplicity of use, the additional weight given to critical locations of psoriasis, the possibility for the patient to self-assess his own disease, and the possibility of evaluating all the dimensions of psoriasis at the same time.
Introduction: Plusieurs scores ont été développés permettant d’évaluer la sévérité du psoriasis. Malgré leur utilisation large, ces scores présentent de nombreuses limites. Le Simplified Psoriasis Index (SPI) est un score de sévérité qui comprend trois variables distinctes : la sévérité actuelle de la maladie (SPI-s), son impact psychosocial (SPI-p), et l’historique de la maladie psoriasique et de ses traitements (SPI-i). Le SPI est décliné en deux versions similaires : l’une est remplie par le médecin (proSPI) et la deuxième par le patient (saSPI).
Objectif : Evaluer la validité du SPI en étudiant sa corrélation aux scores de référence chez les patients tunisiens.
Méthodes :Étude prospective bi-centrique incluant 80 patients atteints de psoriasis en plaques.
Résultats : Le PASI médian était de 7,6. Le DLQI médian était de 9. Le proSPI-s médian était de 6 et le saSPI-s médian était de 8. Le SPI-p médian était de 7. Le SPI-i médian était de 2. Il existait une corrélation forte entre les scores proSPI-s et PASI (r=0,87) et entre les scores proSPI-s et saSPI-s (r=0,82). Il y’avait une corrélation moyenne entre le saSPI-s et le PASI (r=0,70) et entre le SPI-p et le DLQI (r=0,67). La valeur seuil de sévérité du proSPI-s et du saSPI-s était de 7,25. La valeur seuil de sévérité du SPI-p était de 6,5.
Conclusion : Le SPI vise à fournir une mesure concise et globale de sévérité et de l’impact du psoriasis sur la qualité de vie. L’utilisation du SPI présente plusieurs avantages : la simplicité d’utilisation, la pondération supplémentaire accordée aux localisations critiques du psoriasis sur le plan fonctionnel et psychosocial, la possibilité pour le patient de s’autoévaluer, et la possibilité d’évaluer à la fois toutes les dimensions du psoriasis.
Figures



Similar articles
-
Validation of the Simplified Psoriasis Index in Dutch children and adolescents with plaque psoriasis.Br J Dermatol. 2017 Mar;176(3):771-776. doi: 10.1111/bjd.15120. Epub 2016 Dec 8. Br J Dermatol. 2017. PMID: 27718521
-
The Simplified Psoriasis Index (SPI): a practical tool for assessing psoriasis.J Invest Dermatol. 2013 Aug;133(8):1956-62. doi: 10.1038/jid.2013.138. Epub 2013 Mar 20. J Invest Dermatol. 2013. PMID: 23807685 Clinical Trial.
-
Assessing the validity and response distribution of the simplified psoriasis index in patients receiving phototherapy.Australas J Dermatol. 2018 Feb;59(1):41-47. doi: 10.1111/ajd.12549. Epub 2016 Oct 12. Australas J Dermatol. 2018. PMID: 27730628
-
Moderate Psoriasis: A Proposed Definition.Actas Dermosifiliogr. 2017 Dec;108(10):911-917. doi: 10.1016/j.ad.2017.07.002. Epub 2017 Aug 17. Actas Dermosifiliogr. 2017. PMID: 28823420 Review. English, Spanish.
-
Psoriasis severity: commonly used clinical thresholds may not adequately convey patient impact.J Eur Acad Dermatol Venereol. 2021 Feb;35(2):417-421. doi: 10.1111/jdv.16966. Epub 2020 Dec 9. J Eur Acad Dermatol Venereol. 2021. PMID: 32978847
Cited by
-
Pediatric Psoriasis Associated with Van Wyk Grumbach Syndrome: A case report.Tunis Med. 2023 Oct 5;101(10):780-782. Tunis Med. 2023. PMID: 38465761 Free PMC article. English.
-
Associations between diet quality indices and psoriasis severity: results from the Asking People with Psoriasis about Lifestyle and Eating (APPLE) cross-sectional study.Br J Nutr. 2025 Feb 28;133(4):546-557. doi: 10.1017/S0007114525000340. Epub 2025 Feb 20. Br J Nutr. 2025. PMID: 39973353 Free PMC article.
-
Disease severity and demographic factors in relation to subjective well being among syrian psoriasis patients.Sci Rep. 2025 Jul 14;15(1):25407. doi: 10.1038/s41598-025-10328-z. Sci Rep. 2025. PMID: 40659766 Free PMC article.
References
-
- Richard M A, Aractingi S, Joly P. French adaptation of a new score for global assessment of psoriasis severity: The Simplified Psoriasis Index (SPI) Ann Dermatol Venereol. 2019;146:783–92. - PubMed
-
- Finlay Andrew Yule, Khan GK_. Clinical and experimental dermatology. 3. Vol. 19. Wiley Online Library; 1994. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use; pp. 210–216. - PubMed
-
- Naldi Luigi. Clinics in dermatology. 1. Vol. 28. Elsevier; 2010. Scoring and monitoring the severity of psoriasis. What is the preferred method? What is the ideal method? Is PASI passé? facts and controversies; pp. 67–72. - PubMed
-
- Hsu Sylvia, Papp Kim Alexander, Lebwohl Mark G, Bagel Jerry, Blauvelt Andrew, Duffin Kristina Callis, Crowley Jeffrey, Eichenfield Lawrence F, Feldman Steven R, Fiorentino David F. Archives of dermatology. 1. Vol. 148. American Medical Association; 2012. Consensus guidelines for the management of plaque psoriasis; pp. 95–102. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

