Oropharyngeal capsaicin exposure improves infant feeding performance in an animal model of superior laryngeal nerve damage
- PMID: 35822726
- PMCID: PMC9359634
- DOI: 10.1152/jn.00063.2022
Oropharyngeal capsaicin exposure improves infant feeding performance in an animal model of superior laryngeal nerve damage
Abstract
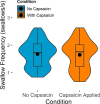
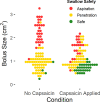
Sensorimotor feedback is critical to safe and effective swallowing. Because of this, sensory interventions have the potential to treat dysphagia. One such treatment may be found in capsaicin, which activates the internal branch of the superior laryngeal nerve (iSLN). The iSLN initiates the pharyngeal swallow, and a more sensitive iSLN should more readily elicit swallowing and improve swallow safety. We explored the neurophysiological mechanism by which capsaicin improves swallow performance using an infant pig model with a unilateral iSLN lesion. Using high-speed videofluoroscopy, we collected oropharyngeal kinematic data while pigs suckled on bottles, before and after applying capsaicin to the posterior tongue and valleculae. We found that capsaicin application decreased maximal bolus sizes, which improved swallow safety. Furthermore, capsaicin improved performance when infant pigs swallowed more moderately sized boluses. However, capsaicin did not change swallow frequency, the number of sucks prior to each swallow, nor total pharyngeal transit time (TPT). Similarly, excursions of the hyoid, thyroid, and posterior tongue were unchanged. TPT and hyoid and thyroid excursions maintained relationships with bolus size post-capsaicin, suggesting that these variables are less sensitive to sensory intervention. The timing and extent of posterior tongue movement were only correlated with bolus size pre-capsaicin, which could imply that capsaicin fundamentally changes in relationships between tongue movements and bolus size. Our results provide insight into the neural control of swallowing and capsaicin's mechanism of action, and suggest that capsaicin may be beneficial in treating acute infant dysphagia.NEW & NOTEWORTHY Chemical sensory interventions alter swallow physiology, which is well-documented in adults but relatively unexplored in infants. Using videofluoroscopy, we found that capsaicin exposure limited infant pigs' bolus sizes to improve swallow performance without changing swallow frequency. Capsaicin increased the likelihood of safe swallowing with more moderately sized boluses and changed relationships between bolus size and tongue movements, which may impact performance. This work highlights the potential role of capsaicin in treating acute infant dysphagia.
Keywords: capsaicin; infant swallowing; kinematics; pediatrics; sensorimotor feedback.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Kandel ER, Schwartz JH. The organization and planning of movement. In: Principles of Neural Science, edited by Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. New York: McGraw Hill Medical, 2013, p. 743–768.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

