Increasing the High Throughput of a Luminescence-Based Serum Bactericidal Assay (L-SBA)
- PMID: 35822773
- PMCID: PMC9245470
- DOI: 10.3390/biotech10030019
Increasing the High Throughput of a Luminescence-Based Serum Bactericidal Assay (L-SBA)
Abstract
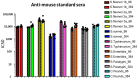
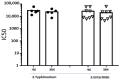
Serum bactericidal assay (SBA) is the method to investigate in vitro complement-mediated bactericidal activity of sera raised upon vaccination. The assay is based on incubating the target bacteria and exogenous complement with sera at different dilutions and the result of the assay is represented by the sera dilution being able to kill 50% of bacteria present in the inoculum. The traditional readout of the assay is based on measurement of colony-forming units (CFU) obtained after plating different reaction mixes on agar. This readout is at low throughput and time consuming, even when automated counting is used. We previously described a novel assay with a luminescence readout (L-SBA) based on measurement of ATP released by live bacteria, which allowed to substantially increase the throughput as well as to reduce the time necessary to perform the assay when compared to traditional methods. Here we present a further improvement of the assay by moving from a 96-well to a 384-well format, which allowed us to further increase the throughput and substantially reduce costs while maintaining the high performance of the previously described L-SBA method. The method has been successfully applied to a variety of different pathogens.
Keywords: functional assay; high throughput; luminescent SBA; serum bactericidal assay; vaccine.
Conflict of interest statement
All authors were employees of the GSK Vaccines Institute for Global Health at the time in which the study was conducted. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA. Some strains used in this study are GSK antigen-producing strains, for which a negotiation with GSK will be necessary in case of material sharing. Other than this, all authors adhere to Journal policies on data and material sharing.
Figures

References
-
- Borrow R., Carlone G.M., Rosenstein N., Blake M., Feavers I., Martin D., Zollinger W., Robbins J., Aaberge I., Granoff D.M., et al. Neisseria meningitidis group B correlates of protection and assay standardization—International meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24:5093–5107. doi: 10.1016/j.vaccine.2006.03.091. - DOI - PubMed
LinkOut - more resources
Full Text Sources
