JIP3 interacts with dynein and kinesin-1 to regulate bidirectional organelle transport
- PMID: 35829703
- PMCID: PMC9284427
- DOI: 10.1083/jcb.202110057
JIP3 interacts with dynein and kinesin-1 to regulate bidirectional organelle transport
Abstract
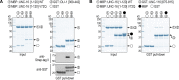
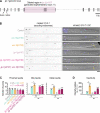
The MAP kinase and motor scaffold JIP3 prevents excess lysosome accumulation in axons of vertebrates and invertebrates. How JIP3's interaction with dynein and kinesin-1 contributes to organelle clearance is unclear. We show that human dynein light intermediate chain (DLIC) binds the N-terminal RH1 domain of JIP3, its paralog JIP4, and the lysosomal adaptor RILP. A point mutation in RH1 abrogates DLIC binding without perturbing the interaction between JIP3's RH1 domain and kinesin heavy chain. Characterization of this separation-of-function mutation in Caenorhabditis elegans shows that JIP3-bound dynein is required for organelle clearance in the anterior process of touch receptor neurons. Unlike JIP3 null mutants, JIP3 that cannot bind DLIC causes prominent accumulation of endo-lysosomal organelles at the neurite tip, which is rescued by a disease-associated point mutation in JIP3's leucine zipper that abrogates kinesin light chain binding. These results highlight that RH1 domains are interaction hubs for cytoskeletal motors and suggest that JIP3-bound dynein and kinesin-1 participate in bidirectional organelle transport.
© 2022 Celestino et al.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

