The changing landscape of immune cells in the fetal mouse testis
- PMID: 35829816
- PMCID: PMC9512757
- DOI: 10.1007/s00418-022-02129-6
The changing landscape of immune cells in the fetal mouse testis
Abstract
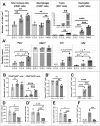
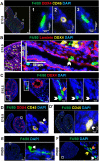
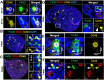
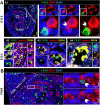
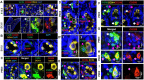
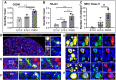
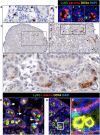
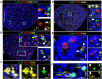
Fetal testis growth involves cell influx and extensive remodeling. Immediately after sex determination in mouse, macrophages enable normal cord formation and removal of inappropriately positioned cells. This study provides new information about macrophages and other immune cells after cord formation in fetal testes, including their density, distribution, and close cellular contacts. C57BL6J mouse testes from embryonic day (E) 13.5 to birth (post-natal day 0; PND0), were examined using immunofluorescence, immunohistochemistry, and RT-qPCR to identify macrophages (F4/80, CD206, MHCII), T cells (CD3), granulocytes/neutrophils (Ly6G), and germ cells (DDX4). F4/80+ cells were the most abundant, comprising 90% of CD45+ cells at E13.5 and declining to 65% at PND0. Changes in size, shape, and markers (CD206 and MHCII) documented during this interval align with the understanding that F4/80+ cells have different origins during embryonic life. CD3+ cells and F4/80-/MHCII+ were absent to rare until PND0. Ly6G+ cells were scarce at E13.5 but increased robustly by PND0 to represent half of the CD45+ cells. These immunofluorescence data were in accord with transcript analysis, which showed that immune marker mRNAs increased with testis age. F4/80+ and Ly6G+ cells were frequently inside cords adjacent to germ cells at E13.5 and E15.5. F4/80+ cells were often in clusters next to other immune cells. Macrophages inside cords at E13.5 and E15.5 (F4/80Hi/CD206+) were different from macrophages at PND0 (F4/80Dim/CD206-), indicating that they have distinct origins. This histological quantification coupled with transcript information identifies new cellular interactions for immune cells in fetal testis morphogenesis, and highlights new avenues for studies of their functional significance.
Keywords: Fetal testis development; Granulocytes; Immune cell localisation; Macrophages; Male germ cells; T cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Testis exposure to unopposed/elevated activin A in utero affects somatic and germ cells and alters steroid levels mimicking phthalate exposure.Front Endocrinol (Lausanne). 2023 Sep 1;14:1234712. doi: 10.3389/fendo.2023.1234712. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37727456 Free PMC article.
-
Role of neurotropins in rat embryonic testis morphogenesis (cord formation).Biol Reprod. 2000 Jan;62(1):132-42. doi: 10.1095/biolreprod62.1.132. Biol Reprod. 2000. PMID: 10611077
-
Testicular immune cell populations and macrophage polarisation in adult male mice and the influence of altered activin A levels.J Reprod Immunol. 2020 Nov;142:103204. doi: 10.1016/j.jri.2020.103204. Epub 2020 Sep 14. J Reprod Immunol. 2020. PMID: 33130539
-
Testis formation in the fetal mouse: dynamic and complex de novo tubulogenesis.Wiley Interdiscip Rev Dev Biol. 2012 Nov-Dec;1(6):847-59. doi: 10.1002/wdev.62. Epub 2012 Apr 26. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23799626 Review.
-
Illuminating testis morphogenesis in the mouse.J Androl. 2010 Jan-Feb;31(1):5-10. doi: 10.2164/jandrol.109.008235. Epub 2009 Oct 29. J Androl. 2010. PMID: 19875491 Review.
Cited by
-
Do macrophages play a role in the adverse effects of endocrine disrupting chemicals (EDCs) on testicular functions?Front Toxicol. 2023 Aug 31;5:1242634. doi: 10.3389/ftox.2023.1242634. eCollection 2023. Front Toxicol. 2023. PMID: 37720385 Free PMC article. Review.
-
Effects of Leydig cell elimination on testicular interstitial cell populations: characterization by scRNA-seq and immunocytochemical techniques.Front Endocrinol (Lausanne). 2024 Aug 20;15:1423801. doi: 10.3389/fendo.2024.1423801. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39229372 Free PMC article.
-
Testis exposure to unopposed/elevated activin A in utero affects somatic and germ cells and alters steroid levels mimicking phthalate exposure.Front Endocrinol (Lausanne). 2023 Sep 1;14:1234712. doi: 10.3389/fendo.2023.1234712. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37727456 Free PMC article.
References
-
- Akhtar M, al-Dayel F, Siegrist K, Ezzat A, Neutrophil-rich Ki-1-positive anaplastic large cell lymphoma presenting as a testicular mass. Mod Pathol. 1996;9(8):812–815. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

