Bioavailability Evaluation of Venetoclax Lower-Strength Tablets and Oral Powder Formulations to Establish Interchangeability with the 100 mg Tablet
- PMID: 35829925
- PMCID: PMC9338003
- DOI: 10.1007/s40261-022-01172-4
Bioavailability Evaluation of Venetoclax Lower-Strength Tablets and Oral Powder Formulations to Establish Interchangeability with the 100 mg Tablet
Abstract
Background and objective: Venetoclax is an approved BCL-2 inhibitor, currently under evaluation in different hematological malignancies in adult and pediatric populations. Venetoclax is available as 10, 50, and 100 mg tablets. To provide an alternative to patients who find taking the commonly prescribed 100 mg tablet a challenge, the interchangeability of lower-strength tablets with the 100 mg tablet was investigated. Additionally, newly developed oral suspension powder formulations to facilitate dosing in pediatrics were evaluated.
Methods: Pharmacokinetic data from 80 healthy female participants from three phase I studies were utilized to evaluate the bioavailability of (1) 10 and 50 mg tablets relative to a 100 mg tablet; (2) 0.72 and 7.2% (drug to total weight) oral powder formulations relative to the 100 mg tablet; and (3) oral powder formulations administered using different vehicles (apple juice, apple sauce, and yogurt) relative to water under fed conditions.
Results: Bioavailability assessments at a 100 mg dose of venetoclax demonstrated bioequivalence across the 10, 50, and 100 mg tablet strengths. Oral powder formulations met the bioequivalence criteria (0.80-1.25) with respect to area under the concentration-time curve to time of the last measurable concentration (AUCt) and to infinite time (AUC∞) but exhibited a slightly lower maximum plasma concentration (Cmax). Exposure-response analyses were utilized to demonstrate that the lower Cmax observed with the powder formulations is not clinically meaningful. The delivery vehicles tested did not affect the bioavailability of venetoclax oral powder formulations.
Conclusions: The smaller-sized tablets (10 and 50 mg) and the newly developed oral powder formulations of venetoclax can be used interchangeably with the 100 mg tablets to improve the patients' experience, while maintaining adequate exposure. CLINICAL TRIALS IDENTIFIERS: NCT01682616, 11 September 2012; NCT02005471, 9 December 2013; NCT02242942, 17 September 2014; NCT02203773, 30 July 2014; NCT02287233, 10 November 2014; NCT02993523, 15 December 2016; NCT03069352, 3 March 2017.
© 2022. The Author(s).
Conflict of interest statement
Mohamed Badawi, Xin Chen, Patrick Marroum, Ahmed A. Suleiman, Sven Mensing, Anette Koenigsdorfer, Julia Teresa Schiele, David Hoffman, Rajeev Menon, and Ahmed Hamed Salem are employees of AbbVie Inc. and may hold AbbVie stock and/or stock options. Divya Samineni is an employee of Genentech and may hold Genentech stock and/or stock options. Tammy Palenski is a former employee of AbbVie and may hold AbbVie stock and/or stock options.
Figures
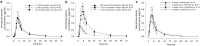

References
-
- AbbVie Inc. Venclexta (venetoclax) [package insert]. US Food and Drug Administration. Revised November 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208573s023lbl.pdf. Accessed Jan 2022.
-
- Alaarg A, Menon R, Rizzo D, Liu Y, Bien J, Elkinton T, et al. A microdosing framework for absolute bioavailability assessment of poorly soluble drugs: a case study on cold-labeled venetoclax, from chemistry to the clinic. Clin Transl Sci. 2022;15(1):244–254. doi: 10.1111/cts.13144. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

