Efficacy and Safety of Topical Nitric Oxide-Releasing Berdazimer Gel in Patients With Molluscum Contagiosum: A Phase 3 Randomized Clinical Trial
- PMID: 35830173
- PMCID: PMC9280611
- DOI: 10.1001/jamadermatol.2022.2721
Efficacy and Safety of Topical Nitric Oxide-Releasing Berdazimer Gel in Patients With Molluscum Contagiosum: A Phase 3 Randomized Clinical Trial
Abstract
Importance: Molluscum contagiosum (MC) is a highly contagious skin condition. Lesions may persist for months to years, and no US Food and Drug Administration-approved medications are currently available in the US.
Objective: To assess the efficacy and safety of berdazimer gel, 10.3%, a novel topical nitric oxide-releasing medication, in the treatment of MC.
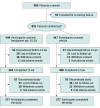
Design, setting, and participants: This was a multicenter, vehicle-controlled, double-blind, phase 3 randomized clinical trial (B-SIMPLE4) conducted in 55 clinics (mostly dermatology and pediatric) in the US from September 1, 2020, to July 21, 2021. Eligible participants were 6 months or older and had from 3 to 70 raised MC lesions. Patients with sexually transmitted MC or with MC only in the periocular area were excluded.
Interventions: Patients were randomized to treatment with berdazimer gel, 10.3%, or vehicle gel, applied as a thin layer to all lesions once daily for 12 weeks.
Main outcomes and measures: The primary efficacy end point was complete clearance of all MC lesions at week 12. Safety and tolerability measures included adverse event frequency and severity, and assessment of local skin reactions and scarring. Data analyses were performed from August 31, 2021, to September 14, 2021.
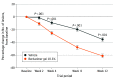
Results: A total of 891 participants were randomized, 444 to berdazimer, 10.3% (mean [range] age, 6.6 [0.9-47.5] years; 228 [51.4%] male; 387 [87.2%] White individuals), and 447 to vehicle (mean [range] age, 6.5 [1.3-49.0] years; 234 [52.3%] female; 382 [85.5%] White individuals). In the intention-to-treat population, 88.5% (393 patients) in the berdazimer group and 88.8% (397 patients) in the vehicle group had a lesion count performed at week 12. At week 12, 32.4% (144 patients) in the berdazimer group achieved complete clearance of MC lesions compared with 19.7% (88 patients) in the vehicle group (absolute difference, 12.7%; odds ratio, 2.0; 95% CI, 1.5-2.8; P < .001) with 14.4% (64 patients) of the berdazimer group discontinuing treatment because of MC clearance compared with 8.9% (40 patients) of the vehicle group. Adverse event rates were low. The most common adverse events were application-site pain and erythema, mostly mild in severity. Adverse events leading to discontinuation affected 4.1% (18 patients) of the berdazimer group and 0.7% (3 patients) of the vehicle group. The most common local skin reaction was mild to moderate erythema.
Conclusions and relevance: Use of berdazimer gel, 10.3%, for MC appears to demonstrate favorable efficacy and safety with low adverse event rates.
Trial registration: ClinicalTrials.gov Identifier: NCT04535531.
Conflict of interest statement
Figures

Comment in
-
Molluscum Contagiosum Therapeutics-New Options May Be Around the Corner.JAMA Dermatol. 2022 Aug 1;158(8):863-864. doi: 10.1001/jamadermatol.2022.2719. JAMA Dermatol. 2022. PMID: 35830172 No abstract available.
-
Neue topische Therapie bei Dellwarzen.MMW Fortschr Med. 2022 Oct;164(17):31. doi: 10.1007/s15006-022-1967-8. MMW Fortschr Med. 2022. PMID: 36198955 Review. German. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

