Nucleosome assembly and disassembly pathways in vitro
- PMID: 35830437
- PMCID: PMC9278766
- DOI: 10.1371/journal.pone.0267382
Nucleosome assembly and disassembly pathways in vitro
Abstract
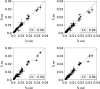
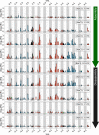
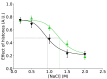
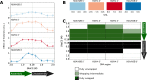
Structural fluctuations of nucleosomes modulate the access to internal DNA in eukaryotic cells; clearly characterisation of this fundamental process is crucial to understanding gene regulation. Here we apply PhAST (Photochemical Analysis of Structural Transitions) to monitor at a base pair level, structural alterations induced all along the DNA upon histone binding or release. By offering the first reliable, detailed comparison of nucleosome assembly and disassembly in vitro, we reveal similarities and differences between the two processes. We identify multiple, sequential intermediate states characterised by specific PhAST signals whose localisation and amplitude reflect asymmetries of DNA/histone interactions with respect to the nucleosome pseudo dyad. These asymmetries involve not only the DNA extremities but also regions close to the pseudo dyad. Localisations of asymmetries develop in a consistent manner during both assembly and disassembly processes; they primarily reflect the DNA sequence effect on the efficiency of DNA-histone binding. More unexpectedly, the amplitude component of PhAST signals not only evolves as a function of intermediate states but does so differently between assembly and disassembly pathways. Our observation of differences between assembly and disassembly opens up new avenues to define the role of the DNA sequence in processes underlying the regulation of gene expression. Overall, we provide new insights into how the intrinsic properties of DNA are integrated into a holistic mechanism that controls chromatin structure.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

