Contribution of individual features to repetition suppression in macaque inferotemporal cortex
- PMID: 35830503
- PMCID: PMC9359640
- DOI: 10.1152/jn.00475.2021
Contribution of individual features to repetition suppression in macaque inferotemporal cortex
Abstract
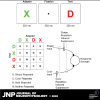
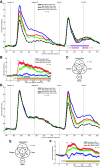
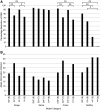
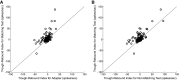
When an image is presented twice in succession, neurons in area TE of macaque inferotemporal cortex exhibit repetition suppression, responding less strongly to the second presentation than to the first. Suppression is known to occur if the adapter and the test image are subtly different from each other. However, it is not known whether cross suppression occurs between images that are radically different from each other but that share a subset of features. To explore this issue, we measured repetition suppression using colored shapes. On interleaved trials, the test image might be identical to the adapter, might share its shape or color alone, or might differ from it totally. At the level of the neuronal population as a whole, suppression was especially deep when adapter and test were identical, intermediate when they shared only one attribute, and minimal when they shared neither attribute. At the level of the individual neuron, the degree of suppression depended not only on the properties of the two images but also on the preferences of the neuron. Suppression was deeper when the repeated color or shape was preferred by the neuron than when it was not. This effect might arise from feature-specific adaptation or alternatively from adapter-induced fatigue. Both mechanisms conform to the principle that the degree of suppression is determined by the preferences of the neuron.NEW & NOTEWORTHY When an image is presented twice in rapid succession, neurons of inferotemporal cortex exhibit repetition suppression, responding less strongly to the second than to the first presentation. It has been unclear whether this phenomenon depends on the selectivity of the neuron under study. Here, we show that, for a given neuron, suppression is deepest when features preferred by that neuron are repeated. The results argue for a mechanism based either on feature-specific suppression or fatigue.
Keywords: inferotemporal cortex; macaque; repetition suppression; rhesus macaque; visual cortex.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






Similar articles
-
Independent repetition suppression in macaque area V2 and inferotemporal cortex.J Neurophysiol. 2022 Dec 1;128(6):1421-1434. doi: 10.1152/jn.00043.2022. Epub 2022 Nov 9. J Neurophysiol. 2022. PMID: 36350050 Free PMC article.
-
Sources of adaptation of inferior temporal cortical responses.Cortex. 2016 Jul;80:185-95. doi: 10.1016/j.cortex.2015.08.024. Epub 2015 Sep 25. Cortex. 2016. PMID: 26518166 Review.
-
Within- and between-hemifield generalization of repetition suppression in inferior temporal cortex.J Neurophysiol. 2021 Jan 1;125(1):120-139. doi: 10.1152/jn.00361.2020. Epub 2020 Nov 11. J Neurophysiol. 2021. PMID: 33174507
-
Probing the Mechanisms of Repetition Suppression in Inferior Temporal Cortex with Optogenetics.Curr Biol. 2019 Jun 17;29(12):1988-1998.e4. doi: 10.1016/j.cub.2019.05.014. Epub 2019 Jun 6. Curr Biol. 2019. PMID: 31178318
-
Single-exposure visual memory judgments are reflected in inferotemporal cortex.Elife. 2018 Mar 8;7:e32259. doi: 10.7554/eLife.32259. Elife. 2018. PMID: 29517485 Free PMC article.
Cited by
-
Independent repetition suppression in macaque area V2 and inferotemporal cortex.J Neurophysiol. 2022 Dec 1;128(6):1421-1434. doi: 10.1152/jn.00043.2022. Epub 2022 Nov 9. J Neurophysiol. 2022. PMID: 36350050 Free PMC article.
-
Temporal dynamics of short-term neural adaptation across human visual cortex.PLoS Comput Biol. 2024 May 30;20(5):e1012161. doi: 10.1371/journal.pcbi.1012161. eCollection 2024 May. PLoS Comput Biol. 2024. PMID: 38815000 Free PMC article.
-
Location-specific neural facilitation in marmoset auditory cortex.Nat Commun. 2025 Mar 20;16(1):2773. doi: 10.1038/s41467-025-58034-8. Nat Commun. 2025. PMID: 40113772 Free PMC article.
-
Temporal continuity shapes visual responses of macaque face patch neurons.Neuron. 2023 Mar 15;111(6):903-914.e3. doi: 10.1016/j.neuron.2022.12.021. Epub 2023 Jan 10. Neuron. 2023. PMID: 36630962 Free PMC article.
References
-
- Saleem KS, Logothetis NK. Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. Amsterdam: Academic Press, 2007.
-
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res 96: 457–472, 1993. doi:10.1007/BF00234113. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

