GOPC facilitates the sorting of syndecan-1 in polarized epithelial cells
- PMID: 35830596
- PMCID: PMC9582621
- DOI: 10.1091/mbc.E22-05-0165
GOPC facilitates the sorting of syndecan-1 in polarized epithelial cells
Abstract
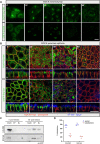
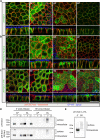
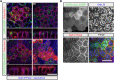
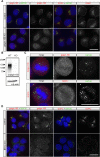
The trans-Golgi network must coordinate sorting and secretion of proteins and lipids to intracellular organelles and the plasma membrane. During polarization of epithelial cells, changes in the lipidome and the expression and distribution of proteins contribute to the formation of apical and basolateral plasma membrane domains. Previous studies using HeLa cells show that the syndecan-1 transmembrane domain confers sorting within sphingomyelin-rich vesicles in a sphingomyelin secretion pathway. In polarized Madin-Darby canine kidney cells, we reveal differences in the sorting of syndecan-1, whereupon the correct trafficking of the protein is not dependent on its transmembrane domain and changes in sphingomyelin content of cells during polarization. Instead, we reveal that correct basolateral targeting of syndecan-1 requires a full-length PDZ motif in syndecan-1 and the PDZ domain golgin protein GOPC. Moreover, we reveal changes in Golgi morphology elicited by GOPC overexpression. These results suggest that the role of GOPC in sorting syndecan-1 is indirect and likely due to GOPC effects on Golgi organization.
Figures

References
-
- Afratis NA, Nikitovic D, Multhaupt HAB, Theocharis AD, Couchman JR, Karamanos NK (2017). Syndecans—key regulators of cell signaling and biological functions. FEBS J 284, 27–41. - PubMed
-
- Boncompain G, Divoux S, Gareil N, de Forges H, Lescure A, Latreche L, Mercanti V, Jollivet F, Raposo G, Perez F (2012). Synchronization of secretory protein traffic in populations of cells. Nat Methods 9, 493–498. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

