VASP localization to lipid bilayers induces polymerization driven actin bundle formation
- PMID: 35830600
- PMCID: PMC9582628
- DOI: 10.1091/mbc.E21-11-0577
VASP localization to lipid bilayers induces polymerization driven actin bundle formation
Abstract
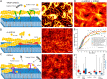
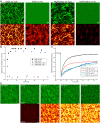
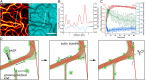
Actin bundles constitute important cytoskeleton structures and enable a scaffold for force transmission inside cells. Actin bundles are formed by proteins, with multiple F-actin binding domains cross-linking actin filaments to each other. Vasodilator-stimulated phosphoprotein (VASP) has mostly been reported as an actin elongator, but it has been shown to be a bundling protein as well and is found in bundled actin structures at filopodia and adhesion sites. Based on in vitro experiments, it remains unclear when and how VASP can act as an actin bundler or elongator. Here we demonstrate that VASP bound to membranes facilitates the formation of large actin bundles during polymerization. The alignment by polymerization requires the fluidity of the lipid bilayers. The mobility within the bilayer enables VASP to bind to filaments and capture and track growing barbed ends. VASP itself phase separates into a protein-enriched phase on the bilayer. This VASP-rich phase nucleates and accumulates at bundles during polymerization, which in turn leads to a reorganization of the underlying lipid bilayer. Our findings demonstrate that the nature of VASP localization is decisive for its function. The up-concentration based on VASP's affinity to actin during polymerization enables it to simultaneously fulfill the function of an elongator and a bundler.
Figures


Similar articles
-
Multiple actin binding domains of Ena/VASP proteins determine actin network stiffening.Eur Biophys J. 2012 Nov;41(11):979-90. doi: 10.1007/s00249-012-0861-1. Epub 2012 Sep 29. Eur Biophys J. 2012. PMID: 23052975
-
VASP protects actin filaments from gelsolin: an in vitro study with implications for platelet actin reorganizations.Cell Motil Cytoskeleton. 2000 Dec;47(4):351-64. doi: 10.1002/1097-0169(200012)47:4<351::AID-CM8>3.0.CO;2-8. Cell Motil Cytoskeleton. 2000. PMID: 11093254 Free PMC article.
-
Reconstitution of actin-based motility by vasodilator-stimulated phosphoprotein (VASP) depends on the recruitment of F-actin seeds from the solution produced by cofilin.J Biol Chem. 2014 Nov 7;289(45):31274-86. doi: 10.1074/jbc.M114.586958. Epub 2014 Sep 22. J Biol Chem. 2014. PMID: 25246528 Free PMC article.
-
Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration.Annu Rev Cell Dev Biol. 2003;19:541-64. doi: 10.1146/annurev.cellbio.19.050103.103356. Annu Rev Cell Dev Biol. 2003. PMID: 14570581 Review.
-
The role of vasodilator-stimulated phosphoprotein in podocyte functioning.Cell Biol Int. 2019 Oct;43(10):1092-1101. doi: 10.1002/cbin.11149. Epub 2019 Jul 22. Cell Biol Int. 2019. PMID: 30968998 Review.
Cited by
-
Liquid-like condensates mediate competition between actin branching and bundling.Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2309152121. doi: 10.1073/pnas.2309152121. Epub 2024 Jan 11. Proc Natl Acad Sci U S A. 2024. PMID: 38207079 Free PMC article.
-
Membrane Ruffles: Composition, Function, Formation and Visualization.Int J Mol Sci. 2024 Oct 12;25(20):10971. doi: 10.3390/ijms252010971. Int J Mol Sci. 2024. PMID: 39456754 Free PMC article. Review.
-
Reconstituted systems for studying the architecture and dynamics of actin networks.Biochem J. 2025 May 23;482(11):691-708. doi: 10.1042/BCJ20253044. Biochem J. 2025. PMID: 40411213 Free PMC article. Review.
-
Benefits and challenges of reconstituting the actin cortex.Cytoskeleton (Hoboken). 2024 Dec;81(12):843-863. doi: 10.1002/cm.21855. Epub 2024 Mar 23. Cytoskeleton (Hoboken). 2024. PMID: 38520148 Review.
-
Actin Bundles Dynamics and Architecture.Biomolecules. 2023 Feb 28;13(3):450. doi: 10.3390/biom13030450. Biomolecules. 2023. PMID: 36979385 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

