Laryngeal and swallow dysregulation following acute cervical spinal cord injury
- PMID: 35830612
- PMCID: PMC9359645
- DOI: 10.1152/jn.00469.2021
Laryngeal and swallow dysregulation following acute cervical spinal cord injury
Abstract
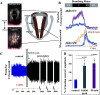
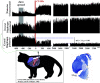
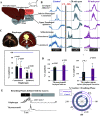
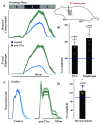
Laryngeal function is vital to airway protection. Although swallow is mediated by the brainstem, the mechanism underlying the increased risk of dysphagia after cervical spinal cord injury (SCI) is unknown. We hypothesized that: 1) loss of descending phrenic drive affects swallow and breathing differently, and 2) loss of ascending spinal afferent information alters swallow regulation. We recorded electromyograms (EMGs) from upper airway and chest wall muscles in freely breathing pentobarbital-anesthetized cats and rats. Laryngeal abductor activity during inspiration increased about twofold following C2 lateral hemisection. Ipsilateral to the injury, the crural diaphragm EMG amplitude was reduced during breathing (62 ± 25% change postinjury), but no animal had complete termination of activity; 75% of animals had increased contralateral diaphragm recruitment, but this did not reach significance. During swallow, laryngeal adductor and pharyngeal constrictor muscles increased activity, and diaphragm activity was bilaterally suppressed. This was unexpected because of the ipsilateral-specific response during breathing. Swallow-breathing coordination was disrupted by injury, and more swallows occurred during early expiration. Finally, to determine if the chest wall is a major source of feedback for laryngeal regulation, we performed T1 total transections in rats. As in the C2 lateral hemisection, inspiratory laryngeal recruitment was the first feature noted after injury. In contrast to the C2 lateral hemisection, diaphragmatic drive increased after T1 transection. Overall, we found that SCI alters laryngeal drive during swallow and breathing, and alters swallow-related diaphragm activity. Our results show behavior-specific effects, suggesting that swallow is affected more than breathing is by SCI, and emphasizing the need for additional studies on the effect of ascending afferents from the spinal cord on laryngeal function.NEW & NOTEWORTHY This is the first manuscript to determine the impact of cSCI on laryngeal and swallow function, and to describe a possible mechanism for dysphagia and altered airway protection after injury.
Keywords: breathing; cervical; larynx; spinal cord injury; swallow.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Impact of glutamatergic and serotonergic neurotransmission on diaphragm muscle activity after cervical spinal hemisection.J Neurophysiol. 2017 Sep 1;118(3):1732-1738. doi: 10.1152/jn.00345.2017. Epub 2017 Jun 28. J Neurophysiol. 2017. PMID: 28659464 Free PMC article.
-
Protein Tyrosine Phosphatase σ Inhibitory Peptide Promotes Recovery of Diaphragm Function and Sprouting of Bulbospinal Respiratory Axons after Cervical Spinal Cord Injury.J Neurotrauma. 2020 Feb 1;37(3):572-579. doi: 10.1089/neu.2019.6586. Epub 2019 Sep 18. J Neurotrauma. 2020. PMID: 31392919 Free PMC article.
-
Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury.J Neurophysiol. 2017 Feb 1;117(2):767-776. doi: 10.1152/jn.00721.2016. Epub 2016 Nov 23. J Neurophysiol. 2017. PMID: 27881723 Free PMC article.
-
Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons.Respir Physiol Neurobiol. 2016 Jun;226:128-36. doi: 10.1016/j.resp.2015.10.009. Epub 2015 Oct 23. Respir Physiol Neurobiol. 2016. PMID: 26506253 Free PMC article. Review.
-
[The clinic experience of implantable diaphragm pacer in a patient with high cervical spinal cord injury and literature review].Zhonghua Jie He He Hu Xi Za Zhi. 2018 Sep 12;41(9):718-723. doi: 10.3760/cma.j.issn.1001-0939.2018.09.013. Zhonghua Jie He He Hu Xi Za Zhi. 2018. PMID: 30196606 Review. Chinese.
Cited by
-
An update on spinal cord injury and diaphragm neuromotor control.Expert Rev Respir Med. 2025 Jul;19(7):679-695. doi: 10.1080/17476348.2025.2495165. Epub 2025 Apr 22. Expert Rev Respir Med. 2025. PMID: 40258801 Review.
-
Deglutition and the Regulation of the Swallow Motor Pattern.Physiology (Bethesda). 2023 Jan 1;38(1):0. doi: 10.1152/physiol.00005.2021. Epub 2022 Aug 23. Physiology (Bethesda). 2023. PMID: 35998250 Free PMC article. Review.
-
Oropharyngeal Dysphagia in Acute Cervical Spinal Cord Injury: A Literature Review.Dysphagia. 2023 Aug;38(4):1025-1038. doi: 10.1007/s00455-022-10535-0. Epub 2022 Nov 14. Dysphagia. 2023. PMID: 36374337 Free PMC article. Review.
-
Clinical Predictors of Dysphagia in Acute and Subacute Traumatic Cervical Spinal Cord Injury: A Retrospective Observational Study.Dysphagia. 2025 Aug 11. doi: 10.1007/s00455-025-10865-9. Online ahead of print. Dysphagia. 2025. PMID: 40784912
-
Stochastic electrical stimulation of the thoracic or cervical regions with surface electrodes facilitates swallow in rats.Front Neurol. 2024 Jul 9;15:1390524. doi: 10.3389/fneur.2024.1390524. eCollection 2024. Front Neurol. 2024. PMID: 39045426 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

