Resolution of R-loops by topoisomerase III-β (TOP3B) in coordination with the DEAD-box helicase DDX5
- PMID: 35830799
- PMCID: PMC10575568
- DOI: 10.1016/j.celrep.2022.111067
Resolution of R-loops by topoisomerase III-β (TOP3B) in coordination with the DEAD-box helicase DDX5
Abstract
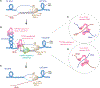
The present study demonstrates how TOP3B is involved in resolving R-loops. We observed elevated R-loops in TOP3B knockout cells (TOP3BKO), which are suppressed by TOP3B transfection. R-loop-inducing agents, the topoisomerase I inhibitor camptothecin, and the splicing inhibitor pladienolide-B also induce higher R-loops in TOP3BKO cells. Camptothecin- and pladienolide-B-induced R-loops are concurrent with the induction of TOP3B cleavage complexes (TOP3Bccs). RNA/DNA hybrid IP-western blotting show that TOP3B is physically associated with R-loops. Biochemical assays using recombinant TOP3B and oligonucleotides mimicking R-loops show that TOP3B cleaves the single-stranded DNA displaced by the R-loop RNA-DNA duplex. IP-mass spectrometry and IP-western experiments reveal that TOP3B interacts with the R-loop helicase DDX5 independently of TDRD3. Finally, we demonstrate that DDX5 and TOP3B are epistatic in resolving R-loops in a pathway parallel with senataxin. We propose a decatenation model for R-loop resolution by TOP3B-DDX5 protecting cells from R-loop-induced damage.
Keywords: CP: Molecular biology; DDX5; DNA-RNA hybrids; DNA/RNA topoisomerase 3B; R-loop; TDRD3; senataxin; topoisomerase cleavage complexes.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

