A specialized Hsp90 co-chaperone network regulates steroid hormone receptor response to ligand
- PMID: 35830801
- PMCID: PMC9306012
- DOI: 10.1016/j.celrep.2022.111039
A specialized Hsp90 co-chaperone network regulates steroid hormone receptor response to ligand
Abstract
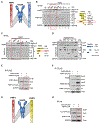
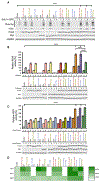
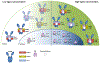
Heat shock protein-90 (Hsp90) chaperone machinery is involved in the stability and activity of its client proteins. The chaperone function of Hsp90 is regulated by co-chaperones and post-translational modifications. Although structural evidence exists for Hsp90 interaction with clients, our understanding of the impact of Hsp90 chaperone function toward client activity in cells remains elusive. Here, we dissect the impact of recently identified higher eukaryotic co-chaperones, FNIP1/2 (FNIPs) and Tsc1, toward Hsp90 client activity. Our data show that Tsc1 and FNIP2 form mutually exclusive complexes with FNIP1, and that unlike Tsc1, FNIP1/2 interact with the catalytic residue of Hsp90. Functionally, these co-chaperone complexes increase the affinity of the steroid hormone receptors glucocorticoid receptor and estrogen receptor to their ligands in vivo. We provide a model for the responsiveness of the steroid hormone receptor activation upon ligand binding as a consequence of their association with specific Hsp90:co-chaperone subpopulations.
Keywords: CP: Molecular biology; FNIP1; FNIP2; Hsp90; Tsc1; androgen receptor; chaperone; chaperone code; co-chaperone; glucocorticoid receptor; steroid hormone receptors.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Abdou NI, Rider V, Greenwell C, Li X, and Kimler BF (2008). Fulvestrant (Faslodex), an estrogen selective receptor downregulator, in therapy of women with systemic lupus erythematosus. clinical, serologic, bone density, and T cell activation marker studies: a double-blind placebo-controlled trial. J. Rheumatol 35, 797. - PubMed
-
- Adams A, Gottschling DE, Kaiser CA, and Stearns T. (1997). Methods in Yeast Genetics (Cold Spring Harbor Laboratory Press; ).
-
- Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, Esposito D, Gillette WK, Hopkins RF 3rd, Hartley JL, et al. (2006). Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc. Natl. Acad. Sci. USA 103, 15552–15557. 10.1073/pnas.0603781103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

