Safety of heterologous primary and booster schedules with ChAdOx1-S and BNT162b2 or mRNA-1273 vaccines: nationwide cohort study
- PMID: 35831006
- PMCID: PMC9277486
- DOI: 10.1136/bmj-2022-070483
Safety of heterologous primary and booster schedules with ChAdOx1-S and BNT162b2 or mRNA-1273 vaccines: nationwide cohort study
Abstract
Objective: To assess the risk of adverse events associated with heterologous primary (two dose) and booster (three dose) vaccine schedules for covid-19 with Oxford-AstraZeneca's ChAdOx1-S priming followed by mRNA vaccines (Pfizer-BioNTech's BNT162b2 or Moderna's mRNA-1273) as compared with homologous mRNA vaccine schedules for covid-19.
Design: Nationwide cohort study.
Setting: Denmark, 1 January 2021 to 26 March 2022.
Participants: Adults aged 18-65 years who received a heterologous vaccine schedule of priming with ChAdOx1-S and one or two mRNA booster doses (with either the BNT162b2 or mRNA-1273 vaccine) were compared with adults who received a homologous BNT162b2 or mRNA-1273 vaccine schedule (ie, two dose v two dose, and three dose v three dose schedule).
Main outcome measures: The incidence of hospital contacts for a range of adverse cardiovascular and haemostatic events within 28 days after the second or third vaccine dose, comparing heterologous versus homologous vaccine schedules. Secondary outcomes included additional prioritised adverse events of special interest. Poisson regression was used to estimate incidence rate ratios with adjustment for selected covariates.
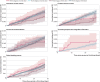
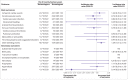
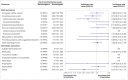
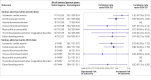
Results: Individuals who had had a heterologous primary vaccine (n=137 495) or a homologous vaccine (n=2 688 142) were identified, in addition to those who had had a heterologous booster (n=129 770) or a homologous booster (n=2 197 213). Adjusted incidence rate ratios of adverse cardiovascular and haemostatic events within 28 days for the heterologous primary and booster vaccine schedules in comparison with the homologous mRNA vaccine schedules were 1.22 (95% confidence interval 0.79 to 1.91) and 1.00 (0.58 to 1.72) for ischaemic cardiac events, 0.74 (0.40 to 1.34) and 0.72 (0.37 to 1.42) for cerebrovascular events, 1.12 (0.13 to 9.58) and 4.74 (0.94 to 24.01) for arterial thromboembolisms, 0.79 (0.45 to 1.38) and 1.09 (0.60 to 1.98) for venous thromboembolisms, 0.84 (0.18 to 3.96) and 1.04 (0.60 to 4.55) for myocarditis or pericarditis, 0.97 (0.45 to 2.10) and 0.89 (0.21 to 3.77) for thrombocytopenia and coagulative disorders, and 1.39 (1.01 to 1.91) and 1.02 (0.70 to 1.47) for other bleeding events, respectively. No associations with any of the outcomes were found when restricting to serious adverse events defined as stay in hospital for more than 24 h.
Conclusion: Heterologous primary and booster covid-19 vaccine schedules of ChAdOx1-S priming and mRNA booster doses as both second and third doses were not associated with increased risk of serious adverse events compared with homologous mRNA vaccine schedules. These results are reassuring but given the rarity of some of the adverse events, associations cannot be excluded.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- European Centre for Disease Prevention and Control. Overview of EU/EEA country recommendations on COVID-19 vaccination with Vaxzevria, and a scoping review of evidence to guide decision-making. 2021.https://www.ecdc.europa.eu/en/publications-data/overview-eueea-country-recommend....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
