Grb2 interacts with necrosome components and is involved in rasfonin-induced necroptosis
- PMID: 35831301
- PMCID: PMC9279413
- DOI: 10.1038/s41420-022-01106-1
Grb2 interacts with necrosome components and is involved in rasfonin-induced necroptosis
Abstract
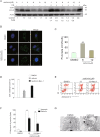
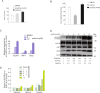
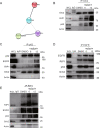
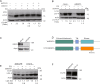
The underlying mechanism by which growth factor receptor-bound protein 2 (Grb2) regulates necroptosis remains unexplored. In the present study, we found that rasfonin, a fungal natural product and an activator of necroptosis, enhanced Grb2 binding to receptor-interacting serine/threonine kinase 1 (RIP1), which plays a critical role in regulating programmed necrosis. Moreover, we observed that SQSTM/p62 (p62), a protein that can form necrosomes with RIP1, increased its interaction with Grb2 upon rasfonin challenge. Although it has been used as an activator of autophagy in our previous study, here we found that a high dose of rasfonin was able to inhibit autophagic process. Inhibition of RIP1 either chemically or genetically reversed the inhibition of rasfonin on autophagy, whereas knockdown of Grb2 markedly reduced rasfonin-induced necrosis. Additionally, we found that the compound failed to upregulate the expression of RIP1 in Grb2-deprived cells. In summary, our data revealed that Grb2 actively participated in rasfonin-induced necroptosis by interacting with the components of necrosome and mediating their expression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ijaz M, Wang FS, Shahbaz M, Jiang WJ, Fathy AH, Nesa EU. The role of Grb2 in cancer and peptides as Grb2 antagonists. Protein Pept Lett. 2017;24:1084–95. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

