Ultrafast-UV laser integrating cavity device for inactivation of SARS-CoV-2 and other viruses
- PMID: 35831374
- PMCID: PMC9279343
- DOI: 10.1038/s41598-022-13670-8
Ultrafast-UV laser integrating cavity device for inactivation of SARS-CoV-2 and other viruses
Abstract
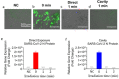
Ultraviolet (UV) irradiation-based methods used for viral inactivation have provided an important avenue targeting severe acute respiratory-syndrome coronavirus-2 (SARS-CoV-2) virus. A major problem with state-of-the-art UV inactivation technology is that it is based on UV lamps, which have limited efficiency, require high power, large doses, and long irradiation times. These drawbacks limit the use of UV lamps in air filtering systems and other applications. To address these limitations, herein we report on the fabrication of a device comprising a pulsed nanosecond 266 nm UV laser coupled to an integrating cavity (LIC) composed of a UV reflective material, polytetrafluoroethylene. Previous UV lamp inactivation cavities were based on polished walls with specular reflections, but the diffuse reflective UV ICs were not thoroughly explored for virus inactivation. Our results show that LIC device can inactivate several respiratory viruses including SARS-CoV-2, at ~ 1 ms effective irradiation time, with > 2 orders of magnitude higher efficiency compared to UV lamps. The demonstrated 3 orders of magnitude cavity enhancement relative to direct exposure is crucial for the development of efficient real-time UV air and water purification systems. To the best of our knowledge this is the first demonstration of LIC application for broad viral inactivation with high efficiency.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Kaushik AK, Dhau JS. Photoelectrochemical oxidation assisted air purifiers; perspective as potential tools to control indoor SARS-CoV-2 exposure. Appl. Surf. Sci. Adv. 2022;9:100236. doi: 10.1016/j.apsadv.2022.100236. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

