Facile fabrication of amino-functionalized MIL-68(Al) metal-organic framework for effective adsorption of arsenate (As(V))
- PMID: 35831402
- PMCID: PMC9279506
- DOI: 10.1038/s41598-022-16038-0
Facile fabrication of amino-functionalized MIL-68(Al) metal-organic framework for effective adsorption of arsenate (As(V))
Abstract
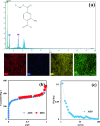
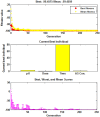
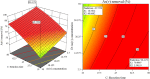
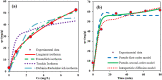
An amino-functionalized MIL-68(Al) metal-organic framework (amino-MIL-68(Al) MOF) was synthesized by solvothermal method and then characterized by FESEM, XRD, FTIR, EDX-mapping, and BET-BJH techniques. In order to predict arsenate (As(V)) removal, a robust quadratic model (R2 > 0.99, F-value = 2389.17 and p value < 0.0001) was developed by the central composite design (CCD) method and then the genetic algorithm (GA) was utilized to optimize the system response and four independent variables. The results showed that As(V) adsorption on MOF was affected by solution pH, adsorbent dose, As(V) concentration and reaction time, respectively. Predicted and experimental As(V) removal efficiencies under optimal conditions were 99.45 and 99.87%, respectively. The fitting of experimental data showed that As(V) adsorption on MOF is well described by the nonlinear form of the Langmuir isotherm and pseudo-second-order kinetic. At optimum pH 3, the maximum As(V) adsorption capacity was 74.29 mg/g. Thermodynamic studies in the temperature range of 25 to 50 °C showed that As(V) adsorption is a spontaneous endothermic process. The reusability of MOF in ten adsorption/regeneration cycles was studied and the results showed high reusability of this adsorbent. The highest interventional effect in inhibiting As(V) adsorption was related to phosphate anion. The results of this study showed that amino-MIL-68(Al) can be used as an effective MOF with a high surface area (> 1000 m2/g) and high reusability for As(V)-contaminated water.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Amen R, Bashir H, Bibi I, Shaheen SM, Niazi NK, Shahid M, Hussain MM, Antoniadis V, Shakoor MB, Al-Solaimani SG, Wang H, Bundschuh J, Rinklebe J. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020;396:125195. doi: 10.1016/j.cej.2020.125195. - DOI
-
- Alka S, Shahir S, Ibrahim N, Ndejiko MJ, Vo D-VN, Abd Manan F. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021;278:123805. doi: 10.1016/j.jclepro.2020.123805. - DOI
-
- Shakoor MB, Niazi NK, Bibi I, Murtaza G, Kunhikrishnan A, Seshadri B, Shahid M, Ali S, Bolan NS, Ok YS. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol. 2016;46:467–499. doi: 10.1080/10643389.2015.1109910. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

