Structure of Tetrahymena telomerase-bound CST with polymerase α-primase
- PMID: 35831498
- PMCID: PMC9728385
- DOI: 10.1038/s41586-022-04931-7
Structure of Tetrahymena telomerase-bound CST with polymerase α-primase
Abstract
Telomeres are the physical ends of linear chromosomes. They are composed of short repeating sequences (such as TTGGGG in the G-strand for Tetrahymena thermophila) of double-stranded DNA with a single-strand 3' overhang of the G-strand and, in humans, the six shelterin proteins: TPP1, POT1, TRF1, TRF2, RAP1 and TIN21,2. TPP1 and POT1 associate with the 3' overhang, with POT1 binding the G-strand3 and TPP1 (in complex with TIN24) recruiting telomerase via interaction with telomerase reverse transcriptase5 (TERT). The telomere DNA ends are replicated and maintained by telomerase6, for the G-strand, and subsequently DNA polymerase α-primase7,8 (PolαPrim), for the C-strand9. PolαPrim activity is stimulated by the heterotrimeric complex CTC1-STN1-TEN110-12 (CST), but the structural basis of the recruitment of PolαPrim and CST to telomere ends remains unknown. Here we report cryo-electron microscopy (cryo-EM) structures of Tetrahymena CST in the context of the telomerase holoenzyme, in both the absence and the presence of PolαPrim, and of PolαPrim alone. Tetrahymena Ctc1 binds telomerase subunit p50, a TPP1 orthologue, on a flexible Ctc1 binding motif revealed by cryo-EM and NMR spectroscopy. The PolαPrim polymerase subunit POLA1 binds Ctc1 and Stn1, and its interface with Ctc1 forms an entry port for G-strand DNA to the POLA1 active site. We thus provide a snapshot of four key components that are required for telomeric DNA synthesis in a single active complex-telomerase-core ribonucleoprotein, p50, CST and PolαPrim-that provides insights into the recruitment of CST and PolαPrim and the handoff between G-strand and C-strand synthesis.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures









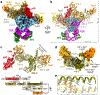



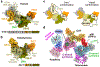
Similar articles
-
Telomere C-Strand Fill-In Machinery: New Insights into the Human CST-DNA Polymerase Alpha-Primase Structures and Functions.Subcell Biochem. 2024;104:73-100. doi: 10.1007/978-3-031-58843-3_5. Subcell Biochem. 2024. PMID: 38963484 Review.
-
POT1 recruits and regulates CST-Polα/primase at human telomeres.Cell. 2024 Jul 11;187(14):3638-3651.e18. doi: 10.1016/j.cell.2024.05.002. Epub 2024 Jun 4. Cell. 2024. PMID: 38838667 Free PMC article.
-
POT1 recruits and regulates CST-Polα/Primase at human telomeres.bioRxiv [Preprint]. 2023 Oct 26:2023.05.08.539880. doi: 10.1101/2023.05.08.539880. bioRxiv. 2023. Update in: Cell. 2024 Jul 11;187(14):3638-3651.e18. doi: 10.1016/j.cell.2024.05.002. PMID: 37215005 Free PMC article. Updated. Preprint.
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
-
Reconstitution of a telomeric replicon organized by CST.Nature. 2022 Aug;608(7924):819-825. doi: 10.1038/s41586-022-04930-8. Epub 2022 Jul 13. Nature. 2022. PMID: 35831508 Free PMC article.
Cited by
-
Models for human telomere C-strand fill-in by CST-Polα-primase.Trends Biochem Sci. 2023 Oct;48(10):860-872. doi: 10.1016/j.tibs.2023.07.008. Epub 2023 Aug 15. Trends Biochem Sci. 2023. PMID: 37586999 Free PMC article. Review.
-
Flexibility and Distributive Synthesis Regulate RNA Priming and Handoff in Human DNA Polymerase α-Primase.J Mol Biol. 2023 Dec 15;435(24):168330. doi: 10.1016/j.jmb.2023.168330. Epub 2023 Oct 24. J Mol Biol. 2023. PMID: 37884206 Free PMC article.
-
Telomere C-Strand Fill-In Machinery: New Insights into the Human CST-DNA Polymerase Alpha-Primase Structures and Functions.Subcell Biochem. 2024;104:73-100. doi: 10.1007/978-3-031-58843-3_5. Subcell Biochem. 2024. PMID: 38963484 Review.
-
Molecular choreography of primer synthesis by the eukaryotic Pol α-primase.Nat Commun. 2023 Jun 21;14(1):3697. doi: 10.1038/s41467-023-39441-1. Nat Commun. 2023. PMID: 37344454 Free PMC article.
-
The yeast CST and Polα/primase complexes act in concert to ensure proper telomere maintenance and protection.Nucleic Acids Res. 2025 Apr 10;53(7):gkaf245. doi: 10.1093/nar/gkaf245. Nucleic Acids Res. 2025. PMID: 40245101 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

