Long-Term Sequelae of COVID-19 in Experimental Mice
- PMID: 35831558
- PMCID: PMC9281331
- DOI: 10.1007/s12035-022-02932-1
Long-Term Sequelae of COVID-19 in Experimental Mice
Abstract
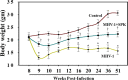
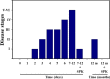
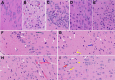
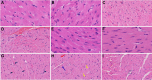
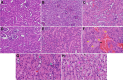
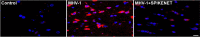
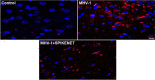
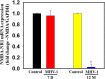
We recently reported acute COVID-19 symptoms, clinical status, weight loss, multi-organ pathological changes, and animal death in a murine hepatitis virus-1 (MHV-1) coronavirus mouse model of COVID-19, which were similar to that observed in humans with COVID-19. We further examined long-term (12 months post-infection) sequelae of COVID-19 in these mice. Congested blood vessels, perivascular cavitation, pericellular halos, vacuolation of neuropils, pyknotic nuclei, acute eosinophilic necrosis, necrotic neurons with fragmented nuclei, and vacuolation were observed in the brain cortex 12 months post-MHV-1 infection. These changes were associated with increased reactive astrocytes and microglia, hyperphosphorylated TDP-43 and tau, and a decrease in synaptic protein synaptophysin-1, suggesting the possible long-term impact of SARS-CoV-2 infection on defective neuronal integrity. The lungs showed severe inflammation, bronchiolar airway wall thickening due to fibrotic remodeling, bronchioles with increased numbers of goblet cells in the epithelial lining, and bronchiole walls with increased numbers of inflammatory cells. Hearts showed severe interstitial edema, vascular congestion and dilation, nucleated red blood cells (RBCs), RBCs infiltrating between degenerative myocardial fibers, inflammatory cells and apoptotic bodies and acute myocyte necrosis, hypertrophy, and fibrosis. Long-term changes in the liver and kidney were less severe than those observed in the acute phase. Noteworthy, the treatment of infected mice with a small molecule synthetic peptide which prevents the binding of spike protein to its respective receptors significantly attenuated disease progression, as well as the pathological changes observed post-long-term infection. Collectively, these findings suggest that COVID-19 may result in long-term, irreversible changes predominantly in the brain, lung, and heart.
Keywords: COVID-19; Long-term sequelae, mice; Mouse hepatitis virus-1; Multi-organ histopathology; SARS-CoV-2; Vascular defect.
© 2022. The Author(s).
Figures





References
-
- Lakhani JD, Kapadia S, Choradiya R, Gill RP, Lakhani SJ. COVID-19 and multiorgan dysfunction syndrome. In: Baddour MM, editor. Fighting the COVID-19 pandemic. IntechOpen; 2021.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

