Targeting mitochondrial metabolism for precision medicine in cancer
- PMID: 35831624
- PMCID: PMC9287557
- DOI: 10.1038/s41418-022-01022-y
Targeting mitochondrial metabolism for precision medicine in cancer
Abstract
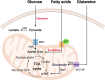
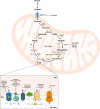
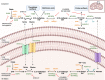
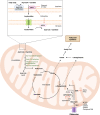
During decades, the research field of cancer metabolism was based on the Warburg effect, described almost one century ago. Lately, the key role of mitochondria in cancer development has been demonstrated. Many mitochondrial pathways including oxidative phosphorylation, fatty acid, glutamine, and one carbon metabolism are altered in tumors, due to mutations in oncogenes and tumor suppressor genes, as well as in metabolic enzymes. This results in metabolic reprogramming that sustains rapid cell proliferation and can lead to an increase in reactive oxygen species used by cancer cells to maintain pro-tumorigenic signaling pathways while avoiding cellular death. The knowledge acquired on the importance of mitochondrial cancer metabolism is now being translated into clinical practice. Detailed genomic, transcriptomic, and metabolomic analysis of tumors are necessary to develop more precise treatments. The successful use of drugs targeting metabolic mitochondrial enzymes has highlighted the potential for their use in precision medicine and many therapeutic candidates are in clinical trials. However, development of efficient personalized drugs has proved challenging and the combination with other strategies such as chemocytotoxic drugs, immunotherapy, and ketogenic or calorie restriction diets is likely necessary to boost their potential. In this review, we summarize the main mitochondrial features, metabolic pathways, and their alterations in different cancer types. We also present an overview of current inhibitors, highlight enzymes that are attractive targets, and discuss challenges with translation of these approaches into clinical practice. The role of mitochondria in cancer is indisputable and presents several attractive targets for both tailored and personalized cancer therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Comment in
-
Mitochondria in the line of fire.Cell Death Differ. 2022 Jul;29(7):1301-1303. doi: 10.1038/s41418-022-01034-8. Epub 2022 Jul 13. Cell Death Differ. 2022. PMID: 35831625 Free PMC article. No abstract available.
References
-
- Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9:148–63. doi: 10.1158/jcr.1925.148. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

