Development and implementation of the AIDA International Registry for patients with Behçet's disease
- PMID: 35831701
- PMCID: PMC9522756
- DOI: 10.1007/s11739-022-03038-1
Development and implementation of the AIDA International Registry for patients with Behçet's disease
Abstract
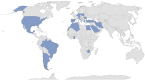
Purpose of the present paper is to point out the design, development and deployment of the AutoInflammatory Disease Alliance (AIDA) International Registry dedicated to pediatric and adult patients with Behçet's disease (BD). The Registry is a clinical physician-driven non-population- and electronic-based instrument implemented for the retrospective and prospective collection of real-life data about demographics, clinical, therapeutic, laboratory, instrumental and socioeconomic information from BD patients; the Registry is based on the Research Electronic Data Capture (REDCap) tool, which is thought to collect standardised information for clinical real-life research, and has been realised to change over time according to future scientific acquisitions and potentially communicate with other existing and future Registries dedicated to BD. Starting from January 31st, 2021, to February 7th, 2022, 110 centres from 23 countries in 4 continents have been involved. Fifty-four of these have already obtained the approval from their local Ethics Committees. Currently, the platform counts 290 users (111 Principal Investigators, 175 Site Investigators, 2 Lead Investigators, and 2 data managers). The Registry collects baseline and follow-up data using 5993 fields organised into 16 instruments, including patient's demographics, history, clinical manifestations and symptoms, trigger/risk factors, therapies and healthcare access. The development of the AIDA International Registry for BD patients will facilitate the collection of standardised data leading to real-world evidence, enabling international multicentre collaborative research through data sharing, international consultation, dissemination of knowledge, inclusion of patients and families, and ultimately optimisation of scientific efforts and implementation of standardised care.Trial registration NCT05200715 in 21/01/2022.
Keywords: Autoinflammatory diseases; Behçet’s disease; International registry; Precision medicine; Rare diseases; Uveitis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment in
-
Registries of rare diseases: current knowledge and future perspectives.Intern Emerg Med. 2023 Jan;18(1):19-21. doi: 10.1007/s11739-022-03151-1. Epub 2022 Nov 19. Intern Emerg Med. 2023. PMID: 36401715 No abstract available.
References
-
- Deliverska M. Patient registries for rare diseases. J IMAB Annu Proc Sci Pap. 2016;22:1166–1168. doi: 10.5272/jimab.2016222.1166. - DOI
-
- Madanat WY, Alawneh KM, Smadi MM, Saadeh SS, Omari MM, Bani Hani AB, et al. The prevalence of Behçet's disease in the north of Jordan: a hospital-based epidemiological survey. Clin Exp Rheumatol. 2017;35:51–54. - PubMed