The gut-liver axis in sepsis: interaction mechanisms and therapeutic potential
- PMID: 35831877
- PMCID: PMC9277879
- DOI: 10.1186/s13054-022-04090-1
The gut-liver axis in sepsis: interaction mechanisms and therapeutic potential
Abstract
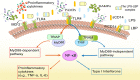
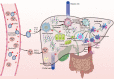
Sepsis is a potentially fatal condition caused by dysregulation of the body's immune response to an infection. Sepsis-induced liver injury is considered a strong independent prognosticator of death in the critical care unit, and there is anatomic and accumulating epidemiologic evidence that demonstrates intimate cross talk between the gut and the liver. Intestinal barrier disruption and gut microbiota dysbiosis during sepsis result in translocation of intestinal pathogen-associated molecular patterns and damage-associated molecular patterns into the liver and systemic circulation. The liver is essential for regulating immune defense during systemic infections via mechanisms such as bacterial clearance, lipopolysaccharide detoxification, cytokine and acute-phase protein release, and inflammation metabolic regulation. When an inappropriate immune response or overwhelming inflammation occurs in the liver, the impaired capacity for pathogen clearance and hepatic metabolic disturbance can result in further impairment of the intestinal barrier and increased disruption of the composition and diversity of the gut microbiota. Therefore, interaction between the gut and liver is a potential therapeutic target. This review outlines the intimate gut-liver cross talk (gut-liver axis) in sepsis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

