Interfering With Contextual Fear Memories by Post-reactivation Administration of Propranolol in Mice: A Series of Null Findings
- PMID: 35832291
- PMCID: PMC9272000
- DOI: 10.3389/fnbeh.2022.893572
Interfering With Contextual Fear Memories by Post-reactivation Administration of Propranolol in Mice: A Series of Null Findings
Abstract
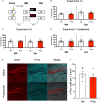
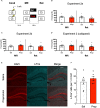
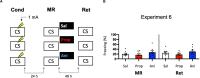
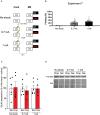
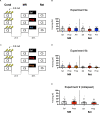
Post-reactivation amnesia of contextual fear memories by blockade of noradrenergic signaling has been shown to have limited replicability in rodents. This is usually attributed to several boundary conditions that gate the destabilization of memory during its retrieval. How these boundary conditions can be overcome, and what neural mechanisms underlie post-reactivation changes in contextual fear memories remain largely unknown. Here, we report a series of experiments in a contextual fear-conditioning paradigm in mice, that were aimed at solving these issues. We first attempted to obtain a training paradigm that would consistently result in contextual fear memory that could be destabilized upon reactivation, enabling post-retrieval amnesia by the administration of propranolol. Unexpectedly, our attempts were unsuccessful to this end. Specifically, over a series of experiments in which we varied different parameters of the fear acquisition procedure, at best small and inconsistent effects were observed. Additionally, we found that propranolol did not alter retrieval-induced neural activity, as measured by the number of c-Fos+ cells in the hippocampal dentate gyrus. To determine whether propranolol was perhaps ineffective in interfering with reactivated contextual fear memories, we also included anisomycin (i.e., a potent and well-known amnesic drug) in several experiments, and measures of synaptic glutamate receptor subunit GluA2 (i.e., a marker of memory destabilization). No post-retrieval amnesia by anisomycin and no altered GluA2 expression by reactivation was observed, suggesting that the memories did not undergo destabilization. The null findings are surprising, given that the training paradigms we implemented were previously shown to result in memories that could be modified upon reactivation. Together, our observations illustrate the elusive nature of reactivation-dependent changes in non-human fear memory.
Keywords: contextual fear; hippocampus; memory reconsolidation; propranolol; rodents.
Copyright © 2022 Cox, Faliagkas, Besseling, van der Loo, Spijker, Kindt and Rao-Ruiz.
Conflict of interest statement
MK has co-founded an outpatient clinic (Kindt Clinics), which offers reconsolidation-based treatments for anxiety disorders. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abrari K., Rashidy-Pour A., Semnanian S., Fathollahi Y. (2008). Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: dependence upon training intensity. Neurobiol. Learn. Mem. 89, 178–184. 10.1016/j.nlm.2007.07.005 - DOI - PubMed
LinkOut - more resources
Full Text Sources

