Parental High-Fat High-Sugar Diet Intake Programming Inflammatory and Oxidative Parameters of Reproductive Health in Male Offspring
- PMID: 35832794
- PMCID: PMC9271829
- DOI: 10.3389/fcell.2022.867127
Parental High-Fat High-Sugar Diet Intake Programming Inflammatory and Oxidative Parameters of Reproductive Health in Male Offspring
Abstract
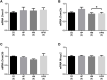
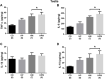
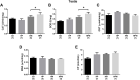
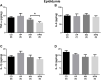
Parental nutrition can impact the health of future generations, programming the offspring for the development of diseases. The developing germ cells of the offspring could be damaged by the maternal or the paternal environment. The germ cells in development and their function could be affected by nutritional adversity and therefore, harm the health of subsequent generations. The paternal or maternal intake of high-fat diets has been shown to affect the reproductive health of male offspring, leading to imbalance in hypothalamic-pituitary-gonadal axis, testicular oxidative stress, low testosterone production, and changes in sperm count, viability, motility, and morphology. There is a need for studies that address the combined effects of diets with a high-fat and high-sugar (H) content by both progenitors on male reproduction. In this context, our study evaluated epigenetic parameters and the inflammatory response that could be associated to oxidative stress in testis and epididymis of adult offspring. 90 days-old male rats were divided according to the combination of the parental diet: CD (control paternal and maternal diet), HP (H paternal diet and control maternal diet), HM (H maternal diet and control paternal diet) and HPM (H paternal and maternal diet).We evaluated serum levels of testosterone and FSH; testicular gene expression of steroidogenic enzymes Star and Hsd17b3 and epigenetic markers Dnmt1, Dnmt3a, Dnmt3b, and Mecp2; testicular and epididymal levels of TNF-α, IL-6, IL-10, and IL-1β; testicular and epididymal activity of SOD, CAT, and GST; the oxidative markers MDA and CP; the daily sperm production, sperm transit time, and sperm morphology. Testicular epigenetic parameter, inflammatory response, oxidative balance, and daily sperm production of the offspring were affected by the maternal diet; paternal diet influenced serum testosterone levels, and lower daily sperm production was exacerbated by the interaction effect of both parental intake of high-fat high-sugar diet in the testis. There was isolated maternal and paternal effect in the antioxidant enzyme activity in the cauda epididymis, and an interaction effect of both parents in protein oxidative marker. Maternal effect could also be observed in cytokine production of cauda epididymis, and no morphological effects were observed in the sperm. The potential programming effects of isolated or combined intake of a high-fat high-sugar diet by the progenitors could be observed at a molecular level in the reproductive health of male offspring in early adulthood.
Keywords: epididimys; epigenetics; fetal programming; high-fat diet; inflammation; oxidative stress; sperm production; testis.
Copyright © 2022 Sertorio, César, de Souza, Mennitti, Santamarina, De Souza Mesquita, Jucá, Casagrande, Estadella, Aguiar and Pisani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

