Development and Application of Transcription Terminators for Polyhydroxylkanoates Production in Halophilic Halomonas bluephagenesis TD01
- PMID: 35832813
- PMCID: PMC9271916
- DOI: 10.3389/fmicb.2022.941306
Development and Application of Transcription Terminators for Polyhydroxylkanoates Production in Halophilic Halomonas bluephagenesis TD01
Abstract
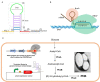
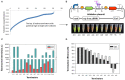
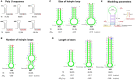
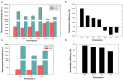
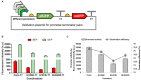
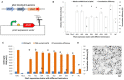
Halomonas bluephagenesis TD01 is one of the ideal chassis for low-cost industrial production based on "Next Generation Industrial Biotechnology," yet the limited genetically regulatory parts such as transcriptional terminators, which are crucial for tuned regulations on gene expression, have hampered the engineering and applications of the strain. In this study, a series of intrinsic Rho-independent terminators were developed by either genome mining or rational design, and seven of them proved to exhibit higher efficiencies than the canonical strong T7 terminator, among which three terminators displayed high efficiencies over 90%. A preliminary modeling on the sequence-efficiency relationship of the terminators suggested that the poly U sequence regularity, the length and GC content of the stem, and the number and the size of hairpin loops remarkably affected the termination efficiency (TE). The rational and de novo designs of novel synthetic terminators based on the sequence-efficiency relationship and the "main contributor" engineering strategy proved to be effective, and fine-tuned polyhydroxylkanoates production was also achieved by the regulation of these native or synthetic terminators with different efficiencies. Furthermore, a perfectly positive correlation between the promoter activity and the TE was revealed in our study. The study enriches our knowledge of transcriptional termination via its sequence-strength relationship and enables the precise regulation of gene expression and PHA synthesis by intrinsic terminators, contributing to the extensive applications of H. bluephagenesis TD01 in the low-cost production of various chemicals.
Keywords: Halomonas bluephagenesis; RNA-Seq; intrinsic terminator; polyhydroxylkanoates; rational design; synthetic biology; termination efficiency.
Copyright © 2022 Xu, Chang, Zhang, Wang, Hong, Zhao, Lu and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cost-Effective Production of L-DOPA by Tyrosinase-Immobilized Polyhydroxyalkanoate Nanogranules in Engineered Halomonas bluephagenesis TD01.Molecules. 2021 Jun 22;26(13):3778. doi: 10.3390/molecules26133778. Molecules. 2021. PMID: 34206459 Free PMC article.
-
Construction of Halomonas bluephagenesis capable of high cell density growth for efficient PHA production.Appl Microbiol Biotechnol. 2018 May;102(10):4499-4510. doi: 10.1007/s00253-018-8931-7. Epub 2018 Apr 5. Appl Microbiol Biotechnol. 2018. PMID: 29623388
-
Promoter Engineering for Enhanced P(3HB- co-4HB) Production by Halomonas bluephagenesis.ACS Synth Biol. 2018 Aug 17;7(8):1897-1906. doi: 10.1021/acssynbio.8b00102. Epub 2018 Jul 31. ACS Synth Biol. 2018. PMID: 30024739
-
Halomonas spp., as chassis for low-cost production of chemicals.Appl Microbiol Biotechnol. 2022 Nov;106(21):6977-6992. doi: 10.1007/s00253-022-12215-3. Epub 2022 Oct 7. Appl Microbiol Biotechnol. 2022. PMID: 36205763 Review.
-
Promoter and Terminator Discovery and Engineering.Adv Biochem Eng Biotechnol. 2018;162:21-44. doi: 10.1007/10_2016_8. Adv Biochem Eng Biotechnol. 2018. PMID: 27277391 Review.
Cited by
-
Establishing Halomonas as a chassis for industrial biotechnology: advances in synthetic biology tool development and metabolic engineering strategies.Microb Cell Fact. 2025 Jun 12;24(1):133. doi: 10.1186/s12934-025-02757-2. Microb Cell Fact. 2025. PMID: 40506695 Free PMC article. Review.
References
-
- Cai L., Tan D., Aibaidula G., Dong X. R., Chen J. C., Tian W. D., et al. . (2011). Comparative genomics study of polyhydroxyalkanoates (PHA) and ectoine relevant genes from Halomonas sp. TD01 revealed extensive horizontal gene transfer events and co-evolutionary relationships. Microb Cell Fact, 10, 88. 10.1186/1475-2859-10-88 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

