Inflammasome activation: from molecular mechanisms to autoinflammation
- PMID: 35832835
- PMCID: PMC9262628
- DOI: 10.1002/cti2.1404
Inflammasome activation: from molecular mechanisms to autoinflammation
Abstract
Inflammasomes are assembled by innate immune sensors that cells employ to detect a range of danger signals and respond with pro-inflammatory signalling. Inflammasomes activate inflammatory caspases, which trigger a cascade of molecular events with the potential to compromise cellular integrity and release the IL-1β and IL-18 pro-inflammatory cytokines. Several molecular mechanisms, working in concert, ensure that inflammasome activation is tightly regulated; these include NLRP3 post-translational modifications, ubiquitination and phosphorylation, as well as single-domain proteins that competitively bind to key inflammasome components, such as the CARD-only proteins (COPs) and PYD-only proteins (POPs). These diverse regulatory systems ensure that a suitable level of inflammation is initiated to counteract any cellular insult, while simultaneously preserving tissue architecture. When inflammasomes are aberrantly activated can drive excessive production of pro-inflammatory cytokines and cell death, leading to tissue damage. In several autoinflammatory conditions, inflammasomes are aberrantly activated with subsequent development of clinical features that reflect the degree of underlying tissue and organ damage. Several of the resulting disease complications may be successfully controlled by anti-inflammatory drugs and/or specific cytokine inhibitors, in addition to more recently developed small-molecule inhibitors. In this review, we will explore the molecular processes underlying the activation of several inflammasomes and highlight their role during health and disease. We also describe the detrimental effects of these inflammasome complexes, in some pathological conditions, and review current therapeutic approaches as well as future prospective treatments.
Keywords: NLRC4; NLRP3; Pyrin; autoinflammatory disorders; inflammasome; inflammation.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
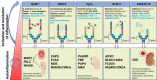
Figures


References
-
- Thaiss CA, Levy M, Itav S, Elinav E. Integration of innate immune signaling. Trends Immunol 2016; 37: 84–101. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL‐beta. Mol Cell 2002; 10: 417–426. - PubMed
-
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016; 16: 407–420. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
