Contribution of septins to human platelet structure and function
- PMID: 35832887
- PMCID: PMC9272382
- DOI: 10.1016/j.isci.2022.104654
Contribution of septins to human platelet structure and function
Abstract
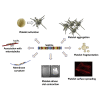
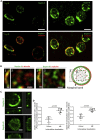
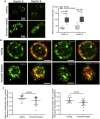
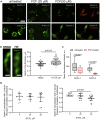
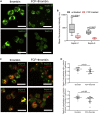
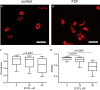
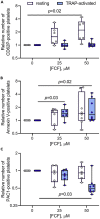
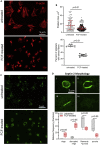
Although septins have been well-studied in nucleated cells, their role in anucleate blood platelets remains obscure. Here, we elucidate the contribution of septins to human platelet structure and functionality. We show that Septin-2 and Septin-9 are predominantly distributed at the periphery of resting platelets and co-localize strongly with microtubules. Activation of platelets by thrombin causes clustering of septins and impairs their association with microtubules. Inhibition of septin dynamics with forchlorfenuron (FCF) reduces thrombin-induced densification of septins and lessens their colocalization with microtubules in resting and activated platelets. Exposure to FCF alters platelet shape, suggesting that septins stabilize platelet cytoskeleton. FCF suppresses platelet integrin αIIbβ3 activation, promotes phosphatidylserine exposure on activated platelets, and induces P-selectin expression on resting platelets, suggesting septin involvement in these processes. Inhibition of septin dynamics substantially reduces platelet contractility and abrogates their spreading on fibrinogen-coated surfaces. Overall, septins strongly contribute to platelet structure, activation and biomechanics.
Keywords: Biological sciences; Cell biology; Functional aspects of cell biology; Integrative aspects of cell biology.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A Septin Cytoskeleton-Targeting Small Molecule, Forchlorfenuron, Inhibits Epithelial Migration via Septin-Independent Perturbation of Cellular Signaling.Cells. 2019 Dec 29;9(1):84. doi: 10.3390/cells9010084. Cells. 2019. PMID: 31905721 Free PMC article.
-
Forchlorfenuron alters mammalian septin assembly, organization, and dynamics.J Biol Chem. 2008 Oct 24;283(43):29563-71. doi: 10.1074/jbc.M804962200. Epub 2008 Aug 18. J Biol Chem. 2008. PMID: 18713753 Free PMC article.
-
Cellular requirements for the small molecule forchlorfenuron to stabilize the septin cytoskeleton.Cytoskeleton (Hoboken). 2010 Jun;67(6):383-99. doi: 10.1002/cm.20452. Cytoskeleton (Hoboken). 2010. PMID: 20517926
-
Role of Septins in Endothelial Cells and Platelets.Front Cell Dev Biol. 2021 Nov 11;9:768409. doi: 10.3389/fcell.2021.768409. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34858990 Free PMC article. Review.
-
The Mammalian Septin Interactome.Front Cell Dev Biol. 2017 Feb 7;5:3. doi: 10.3389/fcell.2017.00003. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28224124 Free PMC article. Review.
Cited by
-
Minor Kinases with Major Roles in Cytokinesis Regulation.Cells. 2022 Nov 17;11(22):3639. doi: 10.3390/cells11223639. Cells. 2022. PMID: 36429067 Free PMC article. Review.
-
Dynamic actin/septin network in megakaryocytes coordinates proplatelet elaboration.Haematologica. 2024 Mar 1;109(3):915-928. doi: 10.3324/haematol.2023.283369. Haematologica. 2024. PMID: 37675512 Free PMC article.
-
Splenectomy improves erythrocyte functionality in spherocytosis based on septin abundance, but not maturation defects.Blood Adv. 2023 Sep 12;7(17):4705-4720. doi: 10.1182/bloodadvances.2022009114. Blood Adv. 2023. PMID: 36753606 Free PMC article.
-
Pharmacological inhibition of Septins with Forchlorfenuron attenuates thrombus formation in experimental thrombotic mice models with modulating multiple signaling pathways in platelets.J Adv Res. 2025 Jul;73:517-533. doi: 10.1016/j.jare.2024.08.006. Epub 2024 Aug 5. J Adv Res. 2025. PMID: 39111626 Free PMC article.
-
Septins as membrane influencers: direct play or in association with other cytoskeleton partners.Front Cell Dev Biol. 2023 Feb 17;11:1112319. doi: 10.3389/fcell.2023.1112319. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36875762 Free PMC article. Review.
References
-
- Ageta-Ishihara N., Miyata T., Ohshima C., Watanabe M., Sato Y., Hamamura Y., Higashiyama T., Mazitschek R., Bito H., Kinoshita M. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat. Commun. 2013;4:2532. doi: 10.1038/ncomms3532. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

