Headache Before and After Endoscopic Transsphenoidal Pituitary Tumor Surgery: A Prospective Study
- PMID: 35832989
- PMCID: PMC9272269
- DOI: 10.1055/s-0041-1729180
Headache Before and After Endoscopic Transsphenoidal Pituitary Tumor Surgery: A Prospective Study
Abstract
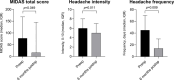
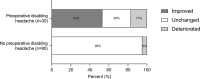
Objective Headache is a common symptom among patients with pituitary tumors, as it is in the general population. The aim of the study was to investigate headache as a symptom in patients with pituitary tumors before and 6 months after endoscopic transsphenoidal surgery (TSS). Design This is a prospective observational cohort study. Setting This study was conducted at university tertiary referral hospital. Participants A total of 110 adult patients underwent endoscopic TSS for pituitary tumors. Main Outcome Measures The Migraine Disability Assessment (MIDAS) questionnaire was used before and 6 months after surgery for the assessment of headache. Clinical variables with potential influence on headache were analyzed. Results Sixty-eight (62%) patients experienced headaches at least once during the 3 months before surgery. Thirty (27%) patients reported disabling headache before surgery, with younger age being an independent associated factor ( p < 0.001). In patients with disabling headache before surgery, the median (interquartile range) MIDAS score improved from 78 (27-168) to 16 (2-145; p = 0.049), headache frequency decreased from 45 (20-81) to 14 (4-35) days ( p = 0.009), and headache intensity decreased from 6 (5-8) to 5 (4-7) ( p = 0.011) after surgery. In total, 16 of the 30 (53%) patients reported a clinically relevant improvement and five (17%) a clinically relevant worsening. Four (5%) patients developed new disabling headache. No predictor for postoperative improvement of headache was identified. Conclusion In this prospective study, the results show that disabling headache improves following endoscopic TSS in a subset of patients with pituitary tumors. However, no predictive factors for improvement could be identified.
Keywords: endoscopic; headache; pituitary tumor; surgery; transsphenoidal.
Thieme. All rights reserved.
Conflict of interest statement
Conflict of Interest T.S. has received lecture fees from Abbott. G.J. has served as consultant for Shire and Astra Zeneca, and has received lecture fees fromEli Lilly, Ipsen, Novartis, Novo Nordisk, Merck Serono, Otsuka, and Pfizer. D.S.O. has served as a consultant for Ipsen, Pfizer, NovoNordisk, and Sandoz, and has received research grants from Sandoz. O.R. has received lecture fees from Novo Nordisk, Ipsen, Sandoz, and Pfizer; an unrestricted research grant from HRA Pharma; and consultancy fees from Novartis, Alnylam, and HRA Pharma.
Figures
References
-
- Levy M J, Jäger H R, Powell M, Matharu M S, Meeran K, Goadsby P J. Pituitary volume and headache: size is not everything. Arch Neurol. 2004;61(05):721–725. - PubMed
-
- Rizzoli P, Iuliano S, Weizenbaum E, Laws E. Headache in patients with pituitary lesions: a longitudinal cohort study. Neurosurgery. 2016;78(03):316–323. - PubMed
-
- Taylor L P. Mechanism of brain tumor headache. Headache. 2014;54(04):772–775. - PubMed
-
- Hayashi Y, Kita D, Iwato M et al. Significant improvement of intractable headache after transsphenoidal surgery in patients with pituitary adenomas; preoperative neuroradiological evaluation and intraoperative intrasellar pressure measurement. Pituitary. 2016;19(02):175–182. - PubMed

