Bacteriological Profile and Antimicrobial Susceptibility Patterns of Gram-Negative Bloodstream Infection and Risk Factors Associated with Mortality and Drug Resistance: A Retrospective Study from Shanxi, China
- PMID: 35833010
- PMCID: PMC9271686
- DOI: 10.2147/IDR.S370326
Bacteriological Profile and Antimicrobial Susceptibility Patterns of Gram-Negative Bloodstream Infection and Risk Factors Associated with Mortality and Drug Resistance: A Retrospective Study from Shanxi, China
Abstract
Objective: The aim of this study was to analyze the epidemiological of gram-negative bloodstream infection (GNBSI) and establish a risk prediction model for mortality and acquiring multidrug resistant (MDR), the extended spectrum beta-lactamases (ESBLs) producing and carbapenem-resistant (CR) GNBSI.
Methods: This retrospective study covered five years from January 2015 to December 2019. Data were obtained from Hospital Information System (HIS) and microbiology department records. The risk factors for mortality and acquiring MDR, ESBLs-producing and CR GNBSI were analyzed by univariable and multivariable analysis.
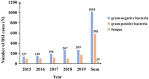
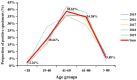
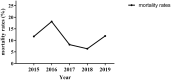
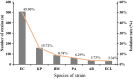
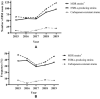
Results: A total of 1018 GNBSI cases were collected. A majority of GNBSI patients were in hematology ward (23.77%). There were 38.61% patients who were assigned in the 41-60 age group. Escherichia coli was the most common gram-negative organism (49.90%). Among isolates of GNBSI, 40.47% were found to be MDR strains, 34.09% were found to be ESBLs-producing strains and 7.06% were found to be CR strains. Escherichia coli was the most common MDR (71.36%) and ESBLs-producing strain (77.81%). Acinetobacter baumannii was the most common CR isolate (46.15%). Multivariate analysis indicated that diabetes mellitus, solid organ tumor, non-fermentative bacteria, MDR strain, central venous cannula, urinary catheter, therapy with carbapenems or tigecycline prior 30 days of infection were independent mortality risk factors for GNBSIs. Over all, therapy with tigecycline prior 30 days of infection was the mutual predictor for mortality of GNBSI, acquiring MDR, ESBLs-producing and CR GNBSI (OR, 8.221, OR, 3.963, OR, 3.588, OR, 9.222, respectively, all p < 0.001).
Conclusion: Collectively, our study implies that patients who were diagnosed as GNBSI had a younger age. Therapy with tigecycline was the mutual and paramount predictor for mortality of GNBSI, acquiring MDR, ESBLs-producing and CR GNBSI. Our investigation had provided a theoretical basis for the use of antibiotics and prevention and control of hospital infection in our region.
Keywords: ESBL; MDR; carbapenem resistance; epidemiology; gram-negative bloodstream infection; risk prediction model.
© 2022 Shi et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Liang T, Xu C, Cheng Q, Tang Y, Zeng H, Li X. Epidemiology, risk factors, and clinical outcomes of bloodstream infection due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in hematologic malignancy: a retrospective study from Central South China. Microb Drug Resist. 2021;27:800–808. doi: 10.1089/mdr.2020.0033 - DOI - PubMed
LinkOut - more resources
Full Text Sources

