Optimizing human coronavirus OC43 growth and titration
- PMID: 35833016
- PMCID: PMC9272819
- DOI: 10.7717/peerj.13721
Optimizing human coronavirus OC43 growth and titration
Abstract
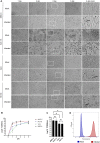
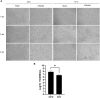
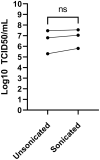
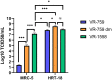
Coronaviruses have been at the forefront of the news for the last 2 years. Unfortunately, SARS-CoV-2, the etiologic agent for the COVID-19 pandemic, must be manipulated in biosecurity level 3 settings, which significantly limits research. Meanwhile, several less pathogenic human coronaviruses (HCoV) exist and can be studied in much more common biosafety level 2 laboratories. Among them, HCoV-OC43 is a good surrogate candidate for SARS-CoV-2 since both are phylogenetically related human Betacoronaviruses. However, one issue has been the lack of standardized means among laboratories to propagate and titer this less virulent coronavirus. The present study probes the optimal parameters to propagate HCoV-OC43. First, testing of five different cell lines (MRC-5, Huh7.5, Vero, HCT-8, HRT-18) indicated that the physiologically relevant MRC-5 human lung cell line produced among the highest viral titers. HRT-18 may however be an interesting alternative as they are quick growing cells that also led to higher viral titers and a better tropism for various HCoV-OC43 variants. We also probed the impact of serum and temperature during viral expansion and confirmed that the normal temperature of the upper respiratory track (33 °C) improves viral yields over the typical 37 °C used to grow many other viruses. Meanwhile, we did not notice any evidence that serum concentrations significantly affected the virus but interestingly noted that the virus grew quite efficiently in a serum-free media formulation. Meanwhile sonication of viral stocks somewhat improved viral titers. Four titration methods (plaque assays, TCID50-CPE, TCID50-IFA and TCID50-IPA) were also probed using two cell lines (VeroE6 and HRT-18). In our hands, plaque assays proved unreliable and quantification of the virus by scoring CPE positive wells was significantly less sensitive than antibody-based assays (IFA and IPA). While the latter methods were equally sensitive, we favor the TCID50-IPA method since simpler, faster and cheaper than the IFA protocol. Moreover, the HRT-18 cells appeared more sensitive to quantify the virus. Perhaps most importantly, these optimized protocols routinely led to high titer viral stocks in the order of 108 TCID50/ml magnitude, which should fulfill the requirements of most experimental settings.
Keywords: COVID; HCoV-OC43; OC43; Propagation; SARS-CoV-2; Titration; Viral stocks.
© 2022 Savoie and Lippé.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Chu H, Chan JF-W, Yuen TT-T, Shuai H, Yuan S, Wang Y, Hu B, Yip CC-Y, Tsang JO-L, Huang X, Chai Y, Yang D, Hou Y, Chik KK-H, Zhang X, Fung AY-F, Tsoi H-W, Cai J-P, Chan W-M, Ip JD, Chu AW-H, Zhou J, Lung DC, Kok K-H, To KK-W, Tsang OT-Y, Chan K-H, Yuen K-Y. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. The Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/S2666-5247(20)30004-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

