Behavioral and Molecular Consequences of Chronic Sleep Restriction During Development in Fragile X Mice
- PMID: 35833085
- PMCID: PMC9271960
- DOI: 10.3389/fnins.2022.834890
Behavioral and Molecular Consequences of Chronic Sleep Restriction During Development in Fragile X Mice
Abstract
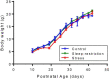
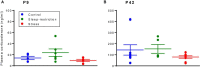
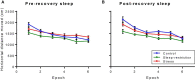
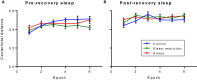
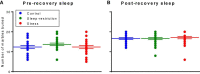
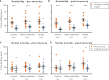
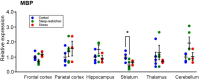
Sleep is critical for brain development and synaptic plasticity. In male wild-type mice, chronic sleep restriction during development results in long-lasting impairments in behavior including hypoactivity, decreased sociability, and increased repetitive behavior. Disordered sleep is characteristic of many neurodevelopmental disorders. Moreover, the severity of behavioral symptoms is correlated with the degree of disordered sleep. We hypothesized that chronic developmental sleep restriction in a mouse model of fragile X syndrome (FXS) would exacerbate behavioral phenotypes. To test our hypothesis, we sleep-restricted Fmr1 knockout (KO) mice for 3 h per day from P5 to P52 and subjected mice to behavioral tests beginning on P42. Contrary to our expectations, sleep restriction improved the hyperactivity and lack of preference for social novelty phenotypes in Fmr1 KO mice but had no measurable effect on repetitive activity. Sleep restriction also resulted in changes in regional distribution of myelin basic protein, suggesting effects on myelination. These findings have implications for the role of disrupted sleep in the severity of symptoms in FXS.
Keywords: autism; chronic sleep restriction; fragile X; gentle handling; mTOR; myelin; social behavior.
Copyright © 2022 Saré, Song, Levine, Lemons, Loutaev, Sheeler, Hildreth, Mfon and Smith.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Chronic sleep restriction during development can lead to long-lasting behavioral effects.Physiol Behav. 2016 Mar 1;155:208-17. doi: 10.1016/j.physbeh.2015.12.019. Epub 2015 Dec 19. Physiol Behav. 2016. PMID: 26712276 Free PMC article.
-
Chronic Sleep Restriction in Developing Male Mice Results in Long Lasting Behavior Impairments.Front Behav Neurosci. 2019 May 3;13:90. doi: 10.3389/fnbeh.2019.00090. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31130852 Free PMC article.
-
Effects of Treatment With Hypnotics on Reduced Sleep Duration and Behavior Abnormalities in a Mouse Model of Fragile X Syndrome.Front Neurosci. 2022 Jun 2;16:811528. doi: 10.3389/fnins.2022.811528. eCollection 2022. Front Neurosci. 2022. PMID: 35720683 Free PMC article.
-
Modeling fragile X syndrome in the Fmr1 knockout mouse.Intractable Rare Dis Res. 2014 Nov;3(4):118-33. doi: 10.5582/irdr.2014.01024. Intractable Rare Dis Res. 2014. PMID: 25606362 Free PMC article. Review.
-
Sensory Processing Phenotypes in Fragile X Syndrome.ASN Neuro. 2018 Jan-Dec;10:1759091418801092. doi: 10.1177/1759091418801092. ASN Neuro. 2018. PMID: 30231625 Free PMC article. Review.
Cited by
-
Loss of sleep when it is needed most - Consequences of persistent developmental sleep disruption: A scoping review of rodent models.Neurobiol Sleep Circadian Rhythms. 2022 Dec 6;14:100085. doi: 10.1016/j.nbscr.2022.100085. eCollection 2023 May. Neurobiol Sleep Circadian Rhythms. 2022. PMID: 36567958 Free PMC article.
References
-
- Frank M. G., Morrissette R., Heller H. C. (1998). Effects of sleep deprivation in neonatal rats. Am. J. Physiol. 275 R148–R157. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

