(-)-Epicatechin Provides Neuroprotection in Sodium Iodate-Induced Retinal Degeneration
- PMID: 35833100
- PMCID: PMC9271623
- DOI: 10.3389/fmed.2022.879901
(-)-Epicatechin Provides Neuroprotection in Sodium Iodate-Induced Retinal Degeneration
Abstract
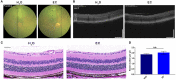
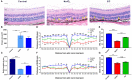
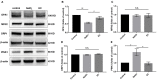
Oxidative stress, mitochondrial impairment, and pathological amyloid beta (Aβ) deposition are involved in the pathogenesis of dry age-related macular degeneration (AMD). The natural flavonoid (-)-epicatechin (EC) is known to be an antioxidant and neuroprotective compound. Whether EC plays a therapeutic role in AMD is unknown. In this work, we aimed to assess the efficacy and molecular mechanisms of EC against sodium iodate (NaIO3)-induced retinal degeneration in C57BL/6 mice via bioinformatic, morphological, and functional methods. We demonstrated that EC had no toxic effects on the retina and could ameliorate retinal deformation and thinning. EC treatment prevented outer retinal degeneration, reduced drusen-like deposits, increased b-wave amplitude in electroretinography, blocked retinal gliosis, and increased the number and quality of mitochondria. Importantly, EC increased the protein expression of OPA1 and decreased the expression of PINK1, indicating the role of EC in mitochondrial fusion that impaired by NaIO3. Moreover, EC downregulated APP and TMEM97 levels, upregulated PGRMC1 levels, and reduced subretinal Aβ accumulation. This study illustrated that EC, which may become a promising therapeutic strategy for AMD, prevented NaIO3-induced retinal degeneration, and this improvement may be associated with the mitochondrial quality control and the TMEM97/PGRMC1/Aβ signaling pathway.
Keywords: (–)-epicatechin; TMEM97; age-related macular degeneration; amyloid beta; mitochondrial dynamics; mitochondrial quality control.
Copyright © 2022 Peng, Zhou, Yao, Li, Song, Xiong and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway.Int J Mol Sci. 2021 Apr 14;22(8):4056. doi: 10.3390/ijms22084056. Int J Mol Sci. 2021. PMID: 33919990 Free PMC article.
-
Protection of retina by αB crystallin in sodium iodate induced retinal degeneration.PLoS One. 2014 May 29;9(5):e98275. doi: 10.1371/journal.pone.0098275. eCollection 2014. PLoS One. 2014. PMID: 24874187 Free PMC article.
-
Glabridin Attenuates the Retinal Degeneration Induced by Sodium Iodate In Vitro and In Vivo.Front Pharmacol. 2020 Oct 15;11:566699. doi: 10.3389/fphar.2020.566699. eCollection 2020. Front Pharmacol. 2020. PMID: 33178017 Free PMC article.
-
Role of amyloid β-peptide in the pathogenesis of age-related macular degeneration.BMJ Open Ophthalmol. 2021 Jun 29;6(1):e000774. doi: 10.1136/bmjophth-2021-000774. eCollection 2021. BMJ Open Ophthalmol. 2021. PMID: 34263061 Free PMC article. Review.
-
Parallel findings in age-related macular degeneration and Alzheimer's disease.Prog Retin Eye Res. 2011 Jul;30(4):217-38. doi: 10.1016/j.preteyeres.2011.02.004. Epub 2011 Mar 25. Prog Retin Eye Res. 2011. PMID: 21440663 Review.
Cited by
-
Co-delivery of antioxidants and siRNA-VEGF: promising treatment for age-related macular degeneration.Drug Deliv Transl Res. 2025 Jul;15(7):2272-2300. doi: 10.1007/s13346-024-01772-x. Epub 2025 Jan 3. Drug Deliv Transl Res. 2025. PMID: 39751765 Review.
References
-
- Khan KN, Mahroo OA, Khan RS, Mohamed MD, McKibbin M, Bird A, et al. . Differentiating drusen: drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog Retin Eye Res. (2016) 53:70–106. 10.1016/j.preteyeres.2016.04.008 - DOI - PubMed
LinkOut - more resources
Full Text Sources

