Improvement Effect of Bifidobacterium animalis subsp. lactis MH-02 in Patients Receiving Resection of Colorectal Polyps: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 35833120
- PMCID: PMC9271559
- DOI: 10.3389/fimmu.2022.940500
Improvement Effect of Bifidobacterium animalis subsp. lactis MH-02 in Patients Receiving Resection of Colorectal Polyps: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Background: Postoperative symptoms, bowel dysfunction and recurrence are common problems after resection of colorectal polyps. We aimed to evaluate the efficacy of Bifidobacterium in the postoperative patients.
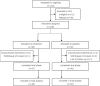
Methods: In this single-center, randomized, double-blind, placebo-controlled trial, adults (≥ 18 years) undergoing endoscopic resection of colorectal polyps were treated with probiotics (Bifidobacterium animalis subsp. lactis MH-02, 2 × 109 colony-forming units per packet) or placebo once daily for 7 days. The primary clinical endpoint was a reduction in the mean total postoperative symptoms score within 7 days postoperatively. Secondary clinical endpoints were the single symptom scores, time to recovery of bowel function, and changes in the intestinal microbiota. This study is registered with the number ChiCTR2100046687.
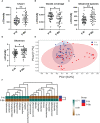
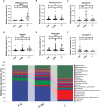
Results: A total of 100 individuals were included (48 in probiotic group and 52 in placebo group). No difference was seen in the mean scores between the two groups (0.29 vs. 0.43, P = 0.246). Colorectal polyps size (P = 0.008) and preoperative symptoms (P = 0.032) were influential factors for the primary endpoint. Besides, MH-02 alleviated difficult defecation (P = 0.045), and reduced the time to recovery of bowel function (P = 0.032). High-throughput analysis showed that MH-02 can help restore the diversity of intestinal microbiota, and increased the relative abundance of Bifidobacterium, Roseburia, Gemmiger, Blautia and Ruminococcus, while reduced the relative abundance of Clostridium at genus level (P < 0.05).
Conclusion: In this prospective trial, MH-02 showed efficacy in patients with resection of colorectal polyps, particularly in the recovery of bowel function, and the changes in the intestinal microbiota may provide evidence for further exploration of the therapeutic mechanisms.
Keywords: Bifidobacterium; MH-02; colorectal polyps; intestinal microbiota; postoperative symptoms.
Copyright © 2022 Liu, Zhang, Liu, Xu, Zhou, Gan, Yao, Li, Chen and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Therapeutic Evaluation of Bifidobacterium animalis subsp. lactis MH-02 as an Adjunctive Treatment in Patients with Reflux Esophagitis: A Randomized, Double-Blind, Placebo-Controlled Trial.Nutrients. 2024 Jan 24;16(3):342. doi: 10.3390/nu16030342. Nutrients. 2024. PMID: 38337627 Free PMC article. Clinical Trial.
-
Bifidobacterium animalis subsp. lactis LPL-RH improves postoperative gastrointestinal symptoms and nutrition indexes by regulating the gut microbiota in patients with valvular heart disease: a randomized controlled trial.Food Funct. 2024 Jul 15;15(14):7605-7618. doi: 10.1039/d4fo01471e. Food Funct. 2024. PMID: 38938120 Clinical Trial.
-
Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: a double-blinded randomized placebo trial.Gut Microbes. 2023 Jan-Dec;15(1):2197837. doi: 10.1080/19490976.2023.2197837. Gut Microbes. 2023. PMID: 37078654 Free PMC article. Clinical Trial.
-
Effects of Bifidobacterium animalis subspecies lactis supplementation on gastrointestinal symptoms: systematic review with meta-analysis.Nutr Rev. 2022 May 9;80(6):1619-1633. doi: 10.1093/nutrit/nuab109. Nutr Rev. 2022. PMID: 34918142
-
THE EFFECT OF PROBIOTIC FERMENTED MILK THAT INCLUDES BIFIDOBACTERIUM LACTIS CNCM I-2494 ON THE REDUCTION OF GASTROINTESTINAL DISCOMFORT AND SYMPTOMS IN ADULTS: A NARRATIVE REVIEW.Nutr Hosp. 2015 Aug 1;32(2):501-9. doi: 10.3305/nh.2015.32.2.9232. Nutr Hosp. 2015. PMID: 26268077 Review.
Cited by
-
Perioperative administration of CBM588 in colorectal cancer radical surgery: A single-center, randomized controlled trial.Cell Rep Med. 2025 Jul 15;6(7):102234. doi: 10.1016/j.xcrm.2025.102234. Epub 2025 Jul 8. Cell Rep Med. 2025. PMID: 40633539 Free PMC article. Clinical Trial.
-
Lactobacillus plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation.Nutrients. 2023 Oct 25;15(21):4512. doi: 10.3390/nu15214512. Nutrients. 2023. PMID: 37960165 Free PMC article.
-
Therapeutic Evaluation of Bifidobacterium animalis subsp. lactis MH-02 as an Adjunctive Treatment in Patients with Reflux Esophagitis: A Randomized, Double-Blind, Placebo-Controlled Trial.Nutrients. 2024 Jan 24;16(3):342. doi: 10.3390/nu16030342. Nutrients. 2024. PMID: 38337627 Free PMC article. Clinical Trial.
-
Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients.Nutrients. 2023 Jan 11;15(2):356. doi: 10.3390/nu15020356. Nutrients. 2023. PMID: 36678227 Free PMC article. Clinical Trial.
-
Alteration in gut microbiota after colonoscopy: proposed mechanisms and the role of probiotic interventions.Clin Endosc. 2025 Jan;58(1):25-39. doi: 10.5946/ce.2024.147. Epub 2024 Sep 2. Clin Endosc. 2025. PMID: 39219335 Free PMC article. Review.
References
-
- Atkin WS, Valori R, Kuipers EJ, Hoff G, Senore C, Segnan N, et al. . European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis. First Edition–Colonoscopic Surveillance Following Adenoma Removal. Endoscopyα (2012) 44 Suppl 3:SE151–63. doi: 10.1055/s-0032-1309821 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

