Type 1 Innate Lymphoid Cells Are Proinflammatory Effector Cells in Ischemia-Reperfusion Injury of Steatotic Livers
- PMID: 35833123
- PMCID: PMC9272906
- DOI: 10.3389/fimmu.2022.899525
Type 1 Innate Lymphoid Cells Are Proinflammatory Effector Cells in Ischemia-Reperfusion Injury of Steatotic Livers
Abstract
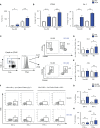
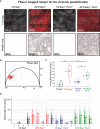
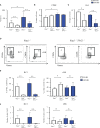
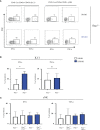
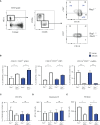
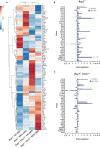
Innate lymphoid cells (ILCs), the most recently described family of lymphoid cells, play fundamental roles in tissue homeostasis through the production of key cytokine. Group 1 ILCs, comprised of conventional natural killer cells (cNKs) and type 1 ILCs (ILC1s), have been implicated in regulating immune-mediated inflammatory diseases. However, the role of ILC1s in nonalcoholic fatty liver disease (NAFLD) and ischemia-reperfusion injury (IRI) is unclear. Here, we investigated the role of ILC1 and cNK cells in a high-fat diet (HFD) murine model of partial warm IRI. We demonstrated that hepatic steatosis results in more severe IRI compared to non-steatotic livers. We further elicited that HFD-IRI mice show a significant increase in the ILC1 population, whereas the cNK population was unchanged. Since ILC1 and cNK are major sources of IFN-γ and TNF-α, we measured the level of ex vivo cytokine expression in normal diet (ND)-IRI and HFD-IRI conditions. We found that ILC1s in HFD-IRI mice produce significantly more IFN-γ and TNF-α when compared to ND-IRI. To further assess whether ILC1s are key proinflammatory effector cells in hepatic IRI of fatty livers, we studied both Rag1-/- mice, which possess cNK cells, and a substantial population of ILC1s versus the newly generated Rag1-/-Tbx21-/- double knockout (Rag1-Tbet DKO) mice, which lack type 1 ILCs, under HFD IRI conditions. Importantly, HFD Rag1-Tbet DKO mice showed significant protection from hepatic injury upon IRI when compared to Rag1-/- mice, suggesting that T-bet-expressing ILC1s play a role, at least in part, as proinflammatory effector cells in hepatic IRI under steatotic conditions.
Keywords: T-bet; Type 1 innate lymphoid cells; fatty liver disease; innate lymphoid cells; ischemia-reperfusion injury; liver transplantation; natural killer cells; phasor fluorescence lifetime imaging.
Copyright © 2022 Kang, Liggett, Patil, Ranjit, Loh, Duttargi, Cui, Oza, Frank, Kwon, Kallakury, Robson, Fishbein, Cui, Khan and Kroemer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Oral N-acetylcysteine decreases IFN-γ production and ameliorates ischemia-reperfusion injury in steatotic livers.Front Immunol. 2022 Sep 5;13:898799. doi: 10.3389/fimmu.2022.898799. eCollection 2022. Front Immunol. 2022. PMID: 36148239 Free PMC article.
-
MMP-9 deficiency shelters endothelial PECAM-1 expression and enhances regeneration of steatotic livers after ischemia and reperfusion injury.J Hepatol. 2014 May;60(5):1032-9. doi: 10.1016/j.jhep.2013.12.022. Epub 2014 Jan 8. J Hepatol. 2014. PMID: 24412604 Free PMC article.
-
Liver type 1 innate lymphoid cells undergo apoptosis in murine models of macrophage activation syndrome and are dispensable for disease.Eur J Immunol. 2024 Dec;54(12):e2451043. doi: 10.1002/eji.202451043. Epub 2024 Sep 30. Eur J Immunol. 2024. PMID: 39348088
-
Type 1 innate lymphoid cells: Soldiers at the front line of immunity.Biomed J. 2021 Apr;44(2):115-122. doi: 10.1016/j.bj.2020.10.001. Epub 2020 Nov 19. Biomed J. 2021. PMID: 33839081 Free PMC article. Review.
-
ILC1s in Tissue Inflammation and Infection.Front Immunol. 2016 Mar 22;7:104. doi: 10.3389/fimmu.2016.00104. eCollection 2016. Front Immunol. 2016. PMID: 27047491 Free PMC article. Review.
Cited by
-
Protective and pathogenic functions of innate lymphoid cells in transplantation.Clin Exp Immunol. 2023 Jul 5;213(1):23-39. doi: 10.1093/cei/uxad050. Clin Exp Immunol. 2023. PMID: 37119279 Free PMC article. Review.
-
Group 1 innate lymphoid cells protect liver transplants from ischemia-reperfusion injury via an interferon gamma-mediated pathway.Am J Transplant. 2025 May;25(5):969-984. doi: 10.1016/j.ajt.2024.11.035. Epub 2024 Dec 28. Am J Transplant. 2025. PMID: 39736469
-
Oral N-acetylcysteine decreases IFN-γ production and ameliorates ischemia-reperfusion injury in steatotic livers.Front Immunol. 2022 Sep 5;13:898799. doi: 10.3389/fimmu.2022.898799. eCollection 2022. Front Immunol. 2022. PMID: 36148239 Free PMC article.
-
Innate immune cells link dietary cues to normal and abnormal metabolic regulation.Nat Immunol. 2025 Jan;26(1):29-41. doi: 10.1038/s41590-024-02037-y. Epub 2025 Jan 2. Nat Immunol. 2025. PMID: 39747429 Free PMC article. Review.
-
Single-cell landscape dissecting the transcription and heterogeneity of innate lymphoid cells in ischemic heart.Front Immunol. 2023 May 9;14:1129007. doi: 10.3389/fimmu.2023.1129007. eCollection 2023. Front Immunol. 2023. PMID: 37228603 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

