Regulatory Roles of Phospholipase A2 Enzymes and Bioactive Lipids in Mast Cell Biology
- PMID: 35833146
- PMCID: PMC9271868
- DOI: 10.3389/fimmu.2022.923265
Regulatory Roles of Phospholipase A2 Enzymes and Bioactive Lipids in Mast Cell Biology
Abstract
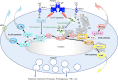
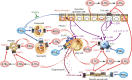
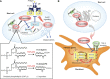
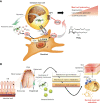
Lipids play fundamental roles in life as an essential component of cell membranes, as a major source of energy, as a body surface barrier, and as signaling molecules that transmit intracellular and intercellular signals. Lipid mediators, a group of bioactive lipids that mediates intercellular signals, are produced via specific biosynthetic enzymes and transmit signals via specific receptors. Mast cells, a tissue-resident immune cell population, produce several lipid mediators that contribute to exacerbation or amelioration of allergic responses and also non-allergic inflammation, host defense, cancer and fibrosis by controlling the functions of microenvironmental cells as well as mast cell themselves in paracrine and autocrine fashions. Additionally, several bioactive lipids produced by stromal cells regulate the differentiation, maturation and activation of neighboring mast cells. Many of the bioactive lipids are stored in membrane phospholipids as precursor forms and released spatiotemporally by phospholipase A2 (PLA2) enzymes. Through a series of studies employing gene targeting and lipidomics, several enzymes belonging to the PLA2 superfamily have been demonstrated to participate in mast cell-related diseases by mobilizing unique bioactive lipids in multiple ways. In this review, we provide an overview of our current understanding of the regulatory roles of several PLA2-driven lipid pathways in mast cell biology.
Keywords: allergy; lipid mediator; mast cells; phospholipase A2; phospholipid; type 2 immunity.
Copyright © 2022 Taketomi and Murakami.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial and financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function.Eur J Pharmacol. 2016 May 5;778:56-67. doi: 10.1016/j.ejphar.2015.02.058. Epub 2015 May 2. Eur J Pharmacol. 2016. PMID: 25941085 Free PMC article. Review.
-
The phospholipase A2 superfamily as a central hub of bioactive lipids and beyond.Pharmacol Ther. 2023 Apr;244:108382. doi: 10.1016/j.pharmthera.2023.108382. Epub 2023 Mar 12. Pharmacol Ther. 2023. PMID: 36918102 Review.
-
[Emerging roles of phospholipase A2s in mast cell biology].Yakugaku Zasshi. 2014;134(11):1179-89. doi: 10.1248/yakushi.14-00198. Yakugaku Zasshi. 2014. PMID: 25366915 Review. Japanese.
-
Liberating Chiral Lipid Mediators, Inflammatory Enzymes, and LIPID MAPS from Biological Grease.J Biol Chem. 2016 Nov 18;291(47):24431-24448. doi: 10.1074/jbc.X116.723791. Epub 2016 Aug 23. J Biol Chem. 2016. PMID: 27555328 Free PMC article. Review.
-
Secreted phospholipase A2 and mast cells.Allergol Int. 2015 Jan;64(1):4-10. doi: 10.1016/j.alit.2014.07.005. Epub 2014 Oct 28. Allergol Int. 2015. PMID: 25572553 Review.
Cited by
-
Deciphering Complex Interactions in Bioactive Lipid Signaling.Molecules. 2023 Mar 14;28(6):2622. doi: 10.3390/molecules28062622. Molecules. 2023. PMID: 36985594 Free PMC article.
-
Differential Mobilization of the Phospholipid and Triacylglycerol Pools of Arachidonic Acid in Murine Macrophages.Biomolecules. 2022 Dec 11;12(12):1851. doi: 10.3390/biom12121851. Biomolecules. 2022. PMID: 36551279 Free PMC article.
-
Dynamics of Docosahexaenoic Acid Utilization by Mouse Peritoneal Macrophages.Biomolecules. 2023 Nov 10;13(11):1635. doi: 10.3390/biom13111635. Biomolecules. 2023. PMID: 38002317 Free PMC article.
-
The Crosstalk with CXCL10-Rich Tumor-Associated Mast Cells Fuels Pancreatic Cancer Progression and Immune Escape.Adv Sci (Weinh). 2025 Apr;12(14):e2417724. doi: 10.1002/advs.202417724. Epub 2025 Feb 18. Adv Sci (Weinh). 2025. PMID: 39965084 Free PMC article.
-
Bioactive lipid signaling and lipidomics in macrophage polarization: Impact on inflammation and immune regulation.Front Immunol. 2025 Feb 14;16:1550500. doi: 10.3389/fimmu.2025.1550500. eCollection 2025. Front Immunol. 2025. PMID: 40028333 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

