Thermal Analyses of Nanowarming-Assisted Recovery of the Heart From Cryopreservation by Vitrification
- PMID: 35833152
- PMCID: PMC8823202
- DOI: 10.1115/1.4053105
Thermal Analyses of Nanowarming-Assisted Recovery of the Heart From Cryopreservation by Vitrification
Abstract
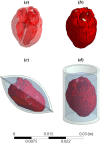
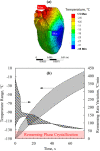
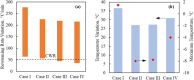
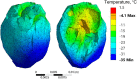
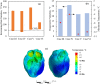
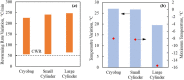
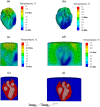
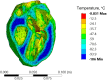
This study explores thermal design aspects of nanowarming-assisted recovery of the heart from indefinite cryogenic storage, where nanowarming is the volumetric heating effect of ferromagnetic nanoparticles excited by a radio frequency electromagnet field. This study uses computational means while focusing on the human heart and the rat heart models. The underlying nanoparticle loading characteristics are adapted from a recent, proof-of-concept experimental study. While uniformly distributed nanoparticles can lead to uniform rewarming, and thereby minimize adverse effects associated with ice crystallization and thermomechanical stress, the combined effects of heart anatomy and nanoparticle loading limitations present practical challenges which this study comes to address. Results of this study demonstrate that under such combined effects, nonuniform nanoparticles warming may lead to a subcritical rewarming rate in some parts of the domain, excessive heating in others, and increased exposure potential to cryoprotective agents (CPAs) toxicity. Nonetheless, the results of this study also demonstrate that computerized planning of the cryopreservation protocol and container design can help mitigate the associated adverse effects, with examples relating to adjusting the CPA and/or nanoparticle concentration, and selecting heart container geometry, and size. In conclusion, nanowarming may provide superior conditions for organ recovery from cryogenic storage under carefully selected conditions, which comes with an elevated complexity of protocol planning and optimization.
Keywords: cryopreservation; nanowarming; simulation; thermal analysis; vitrification.
Copyright © 2022 by ASME.
Figures
Similar articles
-
Vitrification and Rewarming of Magnetic Nanoparticle-Loaded Rat Hearts.Adv Mater Technol. 2022 Mar;7(3):2100873. doi: 10.1002/admt.202100873. Epub 2021 Oct 1. Adv Mater Technol. 2022. PMID: 35668819 Free PMC article.
-
A guide to successful mL to L scale vitrification and rewarming.Cryo Letters. 2022 Nov-Dec;43(6):316-321. Cryo Letters. 2022. PMID: 36629824 Free PMC article. Review.
-
Vitrification and Nanowarming of Kidneys.Adv Sci (Weinh). 2021 Oct;8(19):e2101691. doi: 10.1002/advs.202101691. Epub 2021 Aug 11. Adv Sci (Weinh). 2021. PMID: 34382371 Free PMC article.
-
Thermomechanical Stress in Cryopreservation Via Vitrification With Nanoparticle Heating as a Stress-Moderating Effect.J Biomech Eng. 2016 Jan;138(1). doi: 10.1115/1.4032053. J Biomech Eng. 2016. PMID: 26592974
-
From Nanowarming to Thermoregulation: New Multiscale Applications of Bioheat Transfer.Annu Rev Biomed Eng. 2018 Jun 4;20:301-327. doi: 10.1146/annurev-bioeng-071516-044532. Annu Rev Biomed Eng. 2018. PMID: 29865870 Free PMC article. Review.
Cited by
-
Nanowarming and ice-free cryopreservation of large sized, intact porcine articular cartilage.Commun Biol. 2023 Feb 24;6(1):220. doi: 10.1038/s42003-023-04577-9. Commun Biol. 2023. PMID: 36828843 Free PMC article.
-
Automated device for rapid sample cooling via controlled submersion.Cryobiology. 2025 Jun;119:105250. doi: 10.1016/j.cryobiol.2025.105250. Epub 2025 May 10. Cryobiology. 2025. PMID: 40349384
-
Rapid joule heating improves vitrification based cryopreservation.Nat Commun. 2022 Oct 12;13(1):6017. doi: 10.1038/s41467-022-33546-9. Nat Commun. 2022. PMID: 36224179 Free PMC article.
-
Analysis of crystallization during rewarming in suboptimal vitrification conditions: a semi-empirical approach.Cryobiology. 2021 Dec;103:70-80. doi: 10.1016/j.cryobiol.2021.09.007. Epub 2021 Sep 17. Cryobiology. 2021. PMID: 34543621 Free PMC article.
-
Incorporate delivery, warming and washing methods into efficient cryopreservation.Front Bioeng Biotechnol. 2023 Jun 15;11:1215591. doi: 10.3389/fbioe.2023.1215591. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37397963 Free PMC article. No abstract available.
References
-
- Giwa, S. , Lewis, J. K. , Alvarez, L. , Langer, R. , Roth, A. E. , Church, G. M. , Markmann, J. F. , Sachs, D. H. , Chandraker, A. , Wertheim, J. A. , Rothblatt, M. , Boyden, E. S. , Eidbo, E. , Lee, W. P. A. , Pomahac, B. , Brandacher, G. , Weinstock, D. M. , Elliott, G. , Nelson, D. , Acker, J. P. , Uygun, K. , Schmalz, B. , Weegman, B. P. , Tocchio, A. , Fahy, G. M. , Storey, K. B. , Rubinsky, B. , Bischof, J. , Elliott, J. A. W. , Woodruff, T. K. , Morris, G. J. , Demirci, U. , Brockbank, K. G. M. , Woods, E. J. , Ben, R. N. , Baust, J. G. , Gao, D. , Fuller, B. , Rabin, Y. , Kravitz, D. C. , Taylor, M. J. , and Toner, M. , 2017, “ The Promise of Organ and Tissue Preservation to Transform Medicine,” Nat. Biotechnol., 35(6), pp. 530–542.10.1038/nbt.3889 - DOI - PMC - PubMed
-
- Lewis, J. K. , Bischof, J. C. , Braslavsky, I. , Brockbank, K. G. M. , Fahy, G. M. , Fuller, B. J. , Rabin, Y. , Tocchio, A. , Woods, E. J. , Wowk, B. G. , Acker, J. P. , and Giwa, S. , 2016, “ The Grand Challenges of Organ Banking: Proceedings From the First Global Summit on Complex Tissue Cryopreservation,” Cryobiology, 72(2), pp. 169–182.10.1016/j.cryobiol.2015.12.001 - DOI - PubMed
-
- Campbell, B. K. , Hernandez-Medrano, J. , Onions, V. , Pincott-Allen, C. , Aljaser, F. , Fisher, J. , McNeilly, A. S. , Webb, R. , and Picton, H. M. , 2014, “ Restoration of Ovarian Function and Natural Fertility Following the Cryopreservation and Autotransplantation of Whole Adult Sheep Ovaries,” Hum. Reprod., 29(8), pp. 1749–1763.10.1093/humrep/deu144 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
