Neoadjuvant Trastuzumab and Pertuzumab for Early HER2-Positive Breast Cancer: A Real World Experience
- PMID: 35833190
- PMCID: PMC9262537
- DOI: 10.1155/2022/7146172
Neoadjuvant Trastuzumab and Pertuzumab for Early HER2-Positive Breast Cancer: A Real World Experience
Abstract
Background: Randomized studies of neoadjuvant (NA) trastuzumab and pertuzumab combined with chemotherapy for HER2-positive breast cancers (BC) have reported pathological complete response (pCR) rates of 39 to 61%. This study aimed to determine the real-world efficacy and toxicity of NA trastuzumab and pertuzumab combined with chemotherapy in a UK tertiary referral cancer centre.
Methods: HER2-positive early BC patients given neoadjuvant chemotherapy with trastuzumab and pertuzumab between October 2016 and February 2018 at our tertiary referral cancer centre were identified via pharmacy records. Clinico-pathological information, treatment regimens, treatment-emergent toxicities, operative details, and pathological responses and outcomes were recorded.
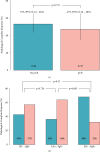
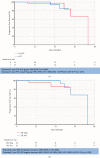
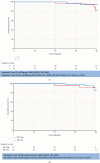
Results: 78 female patients were identified; 2 had bilateral diseases and 48 of 78 (62%) were node positive at presentation. 55 of 80 (71%) tumours were ER-positive. PCR occurred in 37 of 78 (46.3%; 95% CI: 35.3-57.2%) patients. 14 of 23 (60.8%) patients with ER-negative tumours achieved pCR; 23 of 55 (41.8%) were ER-positive and 6 of 19 (31.6%) were ER-positive and PgR-positive. No cardiac toxicity was documented. Diarrhoea occurred in 53 of 72 (74%) patients. Grade 3-4 toxicity occurred in ≥2% patients. These were diarrhoea, fatigue, and infection. The Median follow up period was 45.2 months (95% CI 43.8-46.3) with 71 of 78 (91.0%) remaining disease-free and 72 of 78 (92.3%) alive. Estimated OS at 2 years 86% (95% CI: 75-99%).
Conclusion: This data confirms the efficacy of neoadjuvant chemotherapy combined with dual HER2 directed therapy. While no cardiac toxicity was observed, diarrhoea occurred frequently. The low pCR rate observed in ER and PgR-positive BCs warrants further investigation and consideration of strategies to increase the pCR rate.
Copyright © 2022 Benjamin James Hall et al.
Conflict of interest statement
CP received grant from Pfizer and Daiichi Sankyo and honoraria from Pfizer, Roche, Daiichi Sankyo, Novartis, Exact sciences, Gilead, SeaGen, and Eli Lilly.
Figures
References
-
- Gianni L., Eiermann W., Semiglazov V., et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. The Lancet . 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. - DOI - PubMed
-
- Gianni L., Pienkowski T., Im Y.-H., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (neoSphere): a randomised multicentre, open-label, phase 2 trial. The Lancet Oncology . 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. - DOI - PubMed
-
- Schneeweiss A., Chia S., Hickish T., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Annals of Oncology . 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. - DOI - PubMed
-
- Swain S. M., Ewer M. S., Viale G., et al. Pertuzumab, trastuzumab, and standard anthracycline-and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Annals of Oncology . 2018;29(3):646–653. doi: 10.1093/annonc/mdx773. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

