Carbon dots enhance extracellular matrix secretion for dentin-pulp complex regeneration through PI3K/Akt/mTOR pathway-mediated activation of autophagy
- PMID: 35833197
- PMCID: PMC9272035
- DOI: 10.1016/j.mtbio.2022.100344
Carbon dots enhance extracellular matrix secretion for dentin-pulp complex regeneration through PI3K/Akt/mTOR pathway-mediated activation of autophagy
Abstract
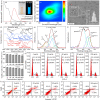
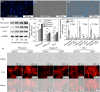
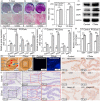
Pulp injury is one of the most common clinical diseases, and severe cases are usually associated with the functional loss of the tooth, while the current clinical treatment modality is only a cavity filling procedure without the regeneration of the dentin-pulp complex, thus leading to a devitalized and brittle tooth. In this study, carbon dots (CDots) with excellent biocompatibility are prepared from ascorbic acid and polyethyleneimine via a hydrothermal method. The as-prepared CDots can enhance extracellular matrix (ECM) secretion of human dental pulp stem cells (DPSCs), giving rise to increased cell adhesion on ECM and a stronger osteogenic/odontogenic differentiation capacity of DPSCs. Further, the mechanism underlying CDots-enhanced ECM secretion is revealed by the transcriptome analysis, Western blot assay and molecular dynamics simulation, identifying that the pharmacological activities of CDots are originated from a reasonable activation of the autophagy, which is mediated by regulating phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Based on the abundant CDots-induced ECM and thereby the reinforcement of the cell-ECM adhesion, an intact dental pulp stem cell sheet can be achieved, which in return promote in vivo the efficient regeneration of dentin-pulp complex as well as blood vessels.
Keywords: Autophagy; Carbon dots; Dentin-pulp complex regeneration; Extracellular matrix; PI3K/Akt/mTOR signaling pathway.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration.Biomaterials. 2015 Jun;52:56-70. doi: 10.1016/j.biomaterials.2015.02.011. Epub 2015 Feb 21. Biomaterials. 2015. PMID: 25818413
-
Biomimetic pulp scaffolds prepared from extracellular matrix derived from stem cells from human exfoliated deciduous teeth promote pulp-dentine complex regeneration.Int Endod J. 2024 Sep;57(9):1279-1292. doi: 10.1111/iej.14099. Epub 2024 Jun 3. Int Endod J. 2024. PMID: 38828966
-
Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration.J Nanobiotechnology. 2024 May 17;22(1):265. doi: 10.1186/s12951-024-02542-0. J Nanobiotechnology. 2024. PMID: 38760763 Free PMC article.
-
Effects of different signaling pathways on odontogenic differentiation of dental pulp stem cells: a review.Front Physiol. 2023 Oct 19;14:1272764. doi: 10.3389/fphys.2023.1272764. eCollection 2023. Front Physiol. 2023. PMID: 37929208 Free PMC article. Review.
-
Hydrogels and Dentin-Pulp Complex Regeneration: From the Benchtop to Clinical Translation.Polymers (Basel). 2020 Dec 9;12(12):2935. doi: 10.3390/polym12122935. Polymers (Basel). 2020. PMID: 33316886 Free PMC article. Review.
Cited by
-
Characterization of a Stemness-Optimized Purification Method for Human Dental-Pulp Stem Cells: An Approach to Standardization.Cells. 2022 Oct 12;11(20):3204. doi: 10.3390/cells11203204. Cells. 2022. PMID: 36291072 Free PMC article.
-
Dentistry Insights: Single-Walled and Multi-Walled Carbon Nanotubes, Carbon Dots, and the Rise of Hybrid Materials.J Funct Biomater. 2025 Mar 20;16(3):110. doi: 10.3390/jfb16030110. J Funct Biomater. 2025. PMID: 40137389 Free PMC article. Review.
-
Carbon dots with tissue engineering and regenerative medicine applications.RSC Adv. 2023 May 15;13(21):14517-14529. doi: 10.1039/d3ra02336b. eCollection 2023 May 9. RSC Adv. 2023. PMID: 37197681 Free PMC article. Review.
-
Functionalized Carbon Dots With Intrinsic Wnt/β-Catenin Inhibition to Synergistically Promote 5-Fluorouracil Chemotherapy.Int J Nanomedicine. 2025 Feb 13;20:1951-1964. doi: 10.2147/IJN.S503540. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39963418 Free PMC article.
-
hsa_circ_0001599 promotes odontogenic differentiation of human dental pulp stem cells by increasing ITGA2 expression and stability.Commun Biol. 2025 Jan 17;8(1):74. doi: 10.1038/s42003-025-07488-z. Commun Biol. 2025. PMID: 39825107 Free PMC article.
References
-
- Peres M.A., Macpherson L.M.D., Weyant R.J., Daly B., Venturelli R., Mathur M.R., Listl S., Celeste R.K., Guarnizo-Herreño C.C., Kearns C., Benzian H., Allison P., Watt R.G. Oral diseases: a global public health challenge. Lancet. 2019;394:249–260. - PubMed
-
- Taubman M.A., Nash D.A. The scientific and public-health imperative for a vaccine against dental caries. Nat. Rev. Immunol. 2006;6:555–563. - PubMed
-
- European Society of Endodontology Quality guidelines for endodontic treatment: consensus report of the european society of endodontology. Int. Endod. J. 2006;39:921–930. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

