Economic burden of severe asthma treatment: A real-life study
- PMID: 35833203
- PMCID: PMC9260620
- DOI: 10.1016/j.waojou.2022.100662
Economic burden of severe asthma treatment: A real-life study
Abstract
Background: Individuals with severe asthma represent 5%-10% of the general asthmatic population. Despite the use of biologic drugs during clinical management, inadequate control of the disease has translated into high economic impact. In Mexico, however, these costs have not yet been assessed.
Methods: A retrospective cohort study was carried out in 2018 and 2019 at Regional Hospital Lic. Adolfo López Mateos, ISSSTE. The assessment of direct costs included pharmacological treatment, clinical tests, days of hospitalization, admissions to the emergency room, and scheduled consultations. The evaluation involved 2 groups of patients-with controlled severe asthma (CSA) and uncontrolled severe asthma (UCSA)-according to presence of exacerbations.
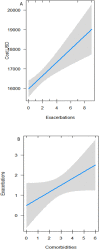
Results: 60 patients (18-75 years old, 51 women) were included in the study. In 2018, 23 of them (38.3%) were categorized as belonging to the UCSA group; in 2019, 22 patients (36.7%) were in this condition (exacerbations: median = 1.5, maximum = 6). Of the 60 patients, 12 (20%) presented between 2 and 9 exacerbations in the study's two-year period (median = 3) after between 4 and 10 years (median = 7.8) of complementary anti-immunoglobulin E (IgE) therapy with omalizumab. The cost for all patients in the 2018-2019 period was 993,289.60 USD. The mean cost per patient was higher for those with UCSA (16,392 USD) than for those with CSA (16,246 USD, p = 0.02). We found a positive association between cost and exacerbations, with an increase of 350 USD per exacerbation (p˂0.0001). Our results indicate that 62% of patients respond to complementary anti-IgE treatment, while 38%-and especially 20%-do not respond optimally to this treatment.
Conclusions: Poor asthma control in this latter group of 38% of patients leads to lower quality of life and higher costs associated with pharmacological treatment.
Keywords: Biological drugs; Cost estimation; Economic burden; Real-life study; Severe asthma.
© 2022 The Author(s).
Figures
References
-
- Rehman A., Amin F., Sadeeqa S. Prevalence of asthma and its management: a review. J Pakistan Med Assoc. 2018;68(12):1823–1827. - PubMed
-
- Wang H., Naghavi M., Allen C., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. - DOI - PMC - PubMed
-
- García-Sancho C., Fernández-Plata R., Martínez-Briseño D., Franco-Marina F., Pérez-Padilla J.R. Prevalencia y riesgos asociados con pacientes adultos con asma de 40 años o más de la Ciudad de México: estudio de base poblacional. Salud Publica Mex. 2012;54(4):425–432. doi: 10.1590/s0036-36342012000400013. - DOI - PubMed
LinkOut - more resources
Full Text Sources