KCl-induced repetitive cortical spreading depression inhibiting trigeminal neuronal firing is mediated by 5-HT1B/1D and opioid receptors
- PMID: 35833238
- PMCID: PMC9638706
- DOI: 10.1177/03331024221112998
KCl-induced repetitive cortical spreading depression inhibiting trigeminal neuronal firing is mediated by 5-HT1B/1D and opioid receptors
Abstract
Background: We aimed to examine the effects of repetitive cortical spreading depression on the responses of nociceptive trigeminal neurons with dural afferents and characterize the role of 5-HT1B/1D and opioid receptors.
Methods: Trigeminocervical complex neurons (n = 53) responsive to nociceptive activation of the dura mater were studied in rats using electrophysiological techniques.
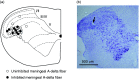
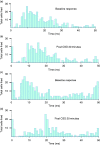
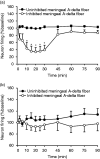
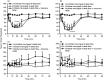
Results: A sub-population (n = 32) showed an average inhibition of dural-evoked responses of 65 ± 14% from baseline with cortical spreading depression. This response was reversed by the selective 5-HT1B/1D receptor antagonist, GR127935 (3 mg/kg; n = 6, iv), and a non-selective opioid receptor antagonist, naloxone (1.5 mg/kg; n = 6, iv), five minutes after injection. To determine the role of the nucleus raphe magnus in the trigeminocervical complex inhibitory effect, microinjection of lidocaine (2%, n = 6) or muscimol (100 mM, n = 5) into the nucleus raphe magnus was performed. There was no effect on cortical spreading depression-induced inhibition of neuronal firing in trigeminocervical complex by either.
Conclusion: The data demonstrate that repetitive cortical spreading depression inhibits a subpopulation of dural nociceptive trigeminocervical neurons, an effect mediated by serotonin and opioid receptors. This inhibition does not involve modulation of nucleus raphe magnus neurons.
Keywords: Cortical spreading depression; migraine; nucleus raphe magnus; opioid; serotonin; trigeminovascular system.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
WS has nothing to report.
PJG reports, over the last 36 months, grants and personal fees from Eli-Lilly and Company, grant from Celgene, and personal fees from Abbvie/Allergan, Aeon Biopharma, Amgen, Biohaven Pharmaceuticals Inc., Dr Reddys, Epalex, Impel Neuropharma, Lundbeck, Novartis, Praxis, Sanofi, Satsuma, Teva Pharmaceuticals, and personal fees from MedicoLegal work, Massachusetts Medical Society, Up-to-Date, Oxford University Press, and Wolters Kluwer; and a patent magnetic stimulation for headache assigned to eNeura without fee.
JH reports honoraria for consulting activities and/or serving on advisory boards from Allergan, Autonomic Technologies Inc., Chordate Medical AB, Eli Lilly, Hormosan Pharma, Novartis and Teva. He received personal fees for Medico-Legal work as well as from Sage Publishing, Springer Healthcare and Quintessence Publishing. All these activities are unrelated to the submitted work.
SA reports personal fees from Allergan, Amgen, GSK, Novartis, and A&O unrelated to the submitted work.
Figures

Similar articles
-
Trigeminocervical complex responses after lesioning dopaminergic A11 nucleus are modified by dopamine and serotonin mechanisms.Pain. 2011 Oct;152(10):2365-2376. doi: 10.1016/j.pain.2011.07.002. Epub 2011 Aug 24. Pain. 2011. PMID: 21868165
-
Serotonin inhibits trigeminal nucleus activity evoked by craniovascular stimulation through a 5HT1B/1D receptor: a central action in migraine?Ann Neurol. 1998 Jun;43(6):711-8. doi: 10.1002/ana.410430605. Ann Neurol. 1998. PMID: 9629840
-
Altered activity in the nucleus raphe magnus underlies cortical hyperexcitability and facilitates trigeminal nociception in a rat model of medication overuse headache.BMC Neurosci. 2019 Oct 21;20(1):54. doi: 10.1186/s12868-019-0536-2. BMC Neurosci. 2019. PMID: 31638891 Free PMC article.
-
Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain.Pain. 2013 Dec;154 Suppl 1:S44-53. doi: 10.1016/j.pain.2013.07.021. Epub 2013 Jul 25. Pain. 2013. PMID: 23891892 Review.
-
Is there a role of calcitonin gene-related peptide in cortical spreading depression mechanisms?- Argument pro.J Headache Pain. 2025 Apr 28;26(1):90. doi: 10.1186/s10194-025-02011-5. J Headache Pain. 2025. PMID: 40295905 Free PMC article. Review. No abstract available.
Cited by
-
Pathophysiology of Migraine: A Disorder of Sensory Processing.Physiol Rev. 2017 Apr;97(2):553-622. doi: 10.1152/physrev.00034.2015. Physiol Rev. 2017. PMID: 28179394 Free PMC article. Review.
References
-
- Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994; 117: 199–210. - PubMed
-
- Charles A. Migraine. N Engl J Med 2017; 377: 553–561. - PubMed
-
- Smith MI, Read SJ, Chan WN, et al.. Repetitive cortical spreading depression in a gyrencephalic feline brain: inhibition by the novel benzoylamino-benzopyran SB-220453. Cephalalgia 2000; 20: 546–553. - PubMed
-
- Goadsby PJ. The oligemic phase of cortical spreading depression is not blocked by tirilazad mesylate (U-74006F). Brain Res 1992; 588: 140–143. - PubMed
-
- Kaube H, Goadsby PJ. Anti-migraine compounds fail to modulate the propagation of cortical spreading depression in the cat. Eur Neurol 1994; 34: 30–35. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

