Human breast milk-derived exosomes through inhibiting AT II cell apoptosis to prevent bronchopulmonary dysplasia in rat lung
- PMID: 35833257
- PMCID: PMC9344832
- DOI: 10.1111/jcmm.17334
Human breast milk-derived exosomes through inhibiting AT II cell apoptosis to prevent bronchopulmonary dysplasia in rat lung
Abstract
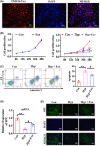
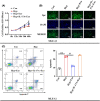
Human breast milk (HBM) effectively prevents and cures neonatal bronchopulmonary dysplasia (BPD). Exosomes are abundant in breast milk, but the function of HBM-derived exosomes (HBM-Exo) in BPD is still unclear. This study was to investigate the role and mechanism of HBM-Exo in BPD. Overall lung tissue photography and H&E staining showed that HBM-Exo improved the lung tissue structure collapse, alveolar structure disorder, alveolar septum width, alveolar number reduction and other injuries caused by high oxygen exposure. Immunohistochemical results showed that HBM-Exo improved the inhibition of cell proliferation and increased apoptosis caused by hyperoxia. qPCR and Western blot results also showed that HBM-Exo improved the expression of Type II alveolar epithelium (AT II) surface marker SPC. In vivo study, CCK8 and flow cytometry showed that HBM-Exo improved the proliferation inhibition and apoptosis of AT II cells induced by hyperoxia, qPCR and immunofluorescence also showed that HBM-Exo improved the down-regulation of SPC. Further RNA-Seq results in AT II cells showed that a total of 88 genes were significantly different between the hyperoxia and HBM-Exo with hyperoxia groups, including 24 up-regulated genes and 64 down-regulated genes. KEGG pathway analysis showed the enrichment of IL-17 signalling pathway was the most significant. Further rescue experiments showed that HBM-Exo improved AT II cell damage induced by hyperoxia through inhibiting downstream of IL-17 signalling pathway (FADD), which may be an important mechanism of HBM-Exo in the prevention and treatment of BPD. This study may provide new approach in the treatment of BPD.
Keywords: AT II; FADD; HBM-Exo; IL-17; apoptosis; bronchopulmonary dysplasia.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
No conflict of interest.
Figures






Similar articles
-
Recruitment of PVT1 Enhances YTHDC1-Mediated m6A Modification of IL-33 in Hyperoxia-Induced Lung Injury During Bronchopulmonary Dysplasia.Inflammation. 2024 Apr;47(2):469-482. doi: 10.1007/s10753-023-01923-1. Epub 2023 Nov 2. Inflammation. 2024. PMID: 37917328 Free PMC article.
-
Adipose mesenchymal stem cells-derived exosomes attenuated hyperoxia-induced lung injury in neonatal rats via inhibiting the NF-κB signaling pathway.Pediatr Pulmonol. 2024 Oct;59(10):2523-2534. doi: 10.1002/ppul.27057. Epub 2024 May 21. Pediatr Pulmonol. 2024. PMID: 38771197
-
Inhibition of microRNA-29a alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice via upregulation of GAB1.Mol Med. 2019 Dec 31;26(1):3. doi: 10.1186/s10020-019-0127-9. Mol Med. 2019. PMID: 31892308 Free PMC article.
-
The Role of Sphingolipid Signaling in Oxidative Lung Injury and Pathogenesis of Bronchopulmonary Dysplasia.Int J Mol Sci. 2022 Jan 23;23(3):1254. doi: 10.3390/ijms23031254. Int J Mol Sci. 2022. PMID: 35163176 Free PMC article. Review.
-
Emerging role of metabolic reprogramming in hyperoxia-associated neonatal diseases.Redox Biol. 2023 Oct;66:102865. doi: 10.1016/j.redox.2023.102865. Epub 2023 Aug 29. Redox Biol. 2023. PMID: 37659187 Free PMC article. Review.
Cited by
-
Removing the stumbling block of exosome applications in clinical and translational medicine: expand production and improve accuracy.Stem Cell Res Ther. 2023 Apr 1;14(1):57. doi: 10.1186/s13287-023-03288-6. Stem Cell Res Ther. 2023. PMID: 37005658 Free PMC article. Review.
-
Therapeutic potential of human breast milk-derived exosomes in necrotizing enterocolitis.Mol Med. 2024 Dec 19;30(1):243. doi: 10.1186/s10020-024-01010-7. Mol Med. 2024. PMID: 39701931 Free PMC article. Review.
-
Extracellular vesicles and preterm infant diseases.Front Pediatr. 2025 Feb 17;13:1550115. doi: 10.3389/fped.2025.1550115. eCollection 2025. Front Pediatr. 2025. PMID: 40034714 Free PMC article. Review.
-
Tat-P combined with GAPR1 releases Beclin1 to promote autophagy and improve Bronchopulmonary dysplasia model.iScience. 2023 Aug 2;26(9):107509. doi: 10.1016/j.isci.2023.107509. eCollection 2023 Sep 15. iScience. 2023. PMID: 37636035 Free PMC article.
-
Monitoring the Use of Human Milk, the Ideal Food for Very Low-Birth-Weight Infants-A Narrative Review.Foods. 2024 Feb 21;13(5):649. doi: 10.3390/foods13050649. Foods. 2024. PMID: 38472762 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

