MiR-483-3p improves learning and memory abilities via XPO1 in Alzheimer's disease
- PMID: 35833267
- PMCID: PMC9392541
- DOI: 10.1002/brb3.2680
MiR-483-3p improves learning and memory abilities via XPO1 in Alzheimer's disease
Abstract
Introduction: Alzheimer's disease (AD), a common form of dementia, has been reported to influence 27 million individuals globally. Several risk factors including oxidative stress, gut microbiota imbalance, and cognitive activity are reported to be closely associated with the initiation or progression of AD. Although miR-483-3p was identified to be downregulated in AD patient serum. However, the biological role and mechanism of miR-483-3p remained unknown in AD. Here, we explored the role of miR-483-3p in AD.
Methods: Sprague-Dawley rats were injected with homocysteine (Hcy) to establish an AD animal model. The Morris water maze tests and contextual fear tests were conducted to assess the cognitive and memory abilities of rats. TUNEL staining was utilized to determine cell apoptosis. Luciferase reporter assay was used to evaluate the binding relation between miR-483-3p and exportin 1 (XPO1).
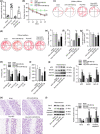
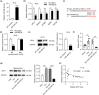
Results: Homocysteine treatment (400 μg/kg) induced the learning, cognitive and memory defects of rats. miR-483-3p was downregulated in Hcy-treated rat hippocampus. Functionally, miR-483-3p alleviated cell apoptosis and impairments of learning and memory abilities in Hcy-treated rats. In addition, miR-483-3p inhibited cell apoptosis and protein level of AD-associated factors (APP, BACE1, and Aβ1-42) in PC12 cells. In mechanism, miR-483-3p was confirmed to target XPO1 in PC12 cells. XPO1 displayed high level in rat hippocampus and was negatively correlated with miR-483-3p levels. Finally, XPO1 overexpression rescued the suppressive effect of miR-483-3p on cell apoptosis and protein levels of AD-associated factors.
Conclusions: miR-483-3p alleviates neural cell apoptosis and impairments of learning and memory abilities by targeting XPO1 in AD.
Keywords: Alzheimer's disease; PC12 cells; XPO1; homocysteine; miR-483-3p.
© 2022 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Figures




Similar articles
-
MicroRNA-132-3p alleviates neuron apoptosis and impairments of learning and memory abilities in Alzheimer's disease by downregulation of HNRNPU stabilized BACE1.Cell Cycle. 2021 Nov;20(21):2309-2320. doi: 10.1080/15384101.2021.1982507. Epub 2021 Sep 29. Cell Cycle. 2021. PMID: 34585626 Free PMC article.
-
miR-29c-3p Attenuates beta-Amyloid-Induced Neurotoxicity in Alzheimer's Disease Through Regulating beta-Site Amyloid Precursor Protein-Cleaving Enzyme 1.Physiol Res. 2023 Dec 31;72(6):833-841. doi: 10.33549/physiolres.935084. Physiol Res. 2023. PMID: 38215068 Free PMC article.
-
Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer's disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway.Aging (Albany NY). 2021 Jun 4;13(11):15285-15306. doi: 10.18632/aging.203088. Epub 2021 Jun 4. Aging (Albany NY). 2021. PMID: 34086603 Free PMC article.
-
miR-212-3p attenuates neuroinflammation of rats with Alzheimer's disease via regulating the SP1/BACE1/NLRP3/Caspase-1 signaling pathway.Bosn J Basic Med Sci. 2022 Jul 29;22(4):540-552. doi: 10.17305/bjbms.2021.6723. Bosn J Basic Med Sci. 2022. PMID: 35150479 Free PMC article.
-
A New Discovery of MicroRNA-455-3p in Alzheimer's Disease.J Alzheimers Dis. 2019;72(s1):S117-S130. doi: 10.3233/JAD-190583. J Alzheimers Dis. 2019. PMID: 31524168 Review.
Cited by
-
Polyphenols and miRNA interplay: a novel approach to combat apoptosis and inflammation in Alzheimer's disease.Front Aging Neurosci. 2025 May 7;17:1571563. doi: 10.3389/fnagi.2025.1571563. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40400913 Free PMC article. Review.
-
CircUTRN24/miR-483-3p/IGF-1 Regulates Autophagy Mediated Liver Fibrosis in Biliary Atresia.Mol Biotechnol. 2024 Jun;66(6):1424-1433. doi: 10.1007/s12033-023-00802-2. Epub 2023 Jun 27. Mol Biotechnol. 2024. PMID: 37369954
-
Regulation of Apoptotic Pathways by MicroRNAs: A Therapeutic Strategy for Alzheimer's Disease.Mol Neurobiol. 2025 Aug;62(8):10577-10613. doi: 10.1007/s12035-025-04833-5. Epub 2025 Apr 12. Mol Neurobiol. 2025. PMID: 40220245 Review.
-
MicroRNA-668-3p regulates oxidative stress and cell damage induced by Aβ1-42 by targeting the OXR1/p53-p21 axis.Ann Transl Med. 2022 Sep;10(17):928. doi: 10.21037/atm-22-3598. Ann Transl Med. 2022. PMID: 36172098 Free PMC article.
-
Exercise-Induced Reduction of IGF1R Sumoylation Attenuates Neuroinflammation in APP/PS1 Transgenic Mice.J Adv Res. 2025 Mar;69:279-297. doi: 10.1016/j.jare.2024.03.025. Epub 2024 Mar 31. J Adv Res. 2025. PMID: 38565402 Free PMC article.
References
-
- Archbold, H. C. , Jackson, K. L. , Arora, A. , Weskamp, K. , Tank, E. M. , Li, X. , Miguez, R. , Dayton, R. D. , Tamir, S. , Klein, R. L. , & Barmada, S. J. (2018). TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Science Reports, 8(1), 4606. 10.1038/s41598-018-22858-w - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

